Abstract
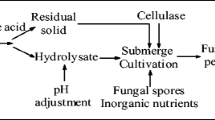
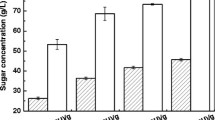
Corncob waste management has become a concern for corn producers worldwide. Agricultural waste-based energy generation is, therefore, extensively researched these days. However, the calorific potential of the moist corncobs still remains untapped due to the lack of technological advancements. In this study, a dilute acid pre-treatment followed with enzymatic hydrolysis was used to recover 80% of the fermentable sugars from the corncobs. When used for microbial oil production in a two-stage fermentation, this enzymatic hydrolysate resulted in a lipid production of more than 75% dried cell weight (DCW) in Rhodotorula glutinis. Extracellular oil capture in this two-stage fermentation further increased the total oil production to 8 g L−1 and 80% DCW. An oleic acid-rich microbial oil profile obtained from this process exhibited excellent biofuel properties. The energy content of the microbial oil valued 40 MJ kg−1, and was found to be 2.35 times higher than the intrinsic heating capacity of corncobs. Thus, a sustainable bioprocess for corncob waste remediation was devised to obtain vegetable oil equivalent through a microbial conversion platform.



Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- ASTM:
-
American Standard for Testing Material
- CFPP:
-
cold filter plugging point
- CN:
-
cetane number
- C/N:
-
ratio of carbon to nitrogen in the media
- CV:
-
calorific value
- DCW:
-
dry cell weight
- FAME:
-
fatty acid methyl esters
- HHV:
-
high heating value
- IV:
-
iodine value
- KV:
-
kinematic viscosity
- LCSF:
-
long-chain saturation factor
- LLE:
-
liquid-liquid extraction
- MUFA:
-
monounsaturated fatty acid
- OCA:
-
oil capturing agent
- PUFA:
-
polyunsaturated fatty acid
- RPR:
-
residue production ratio
- SV:
-
saponification value
- TAG:
-
triacylglycerol
- vvm:
-
volume per volume per minute
References
Hiloidhari M, Das D, Baruah DC (2014) Bioenergy potential from crop residue biomass in India. Renew Sust Energ Rev 32:504–512
Bray CD, Battye WH, Aneja VP (2019) The role of biomass burning agricultural emissions in the Indo-Gangetic Plains on the air quality in New Delhi, India. Atmos Environ 218:116983
Friedl A, Padouvas E, Rotter H, Varmuza K (2005) Prediction of heating values of biomass fuel from elemental composition. Anal Chim Acta 544:191–198
Spalvins K, Vamza I, Blumberga D (2019) Single cell oil production from waste biomass: review of applicable industrial by-products. Environ Clim Technol 23:325–337
Liu Y, Wang Y, Liu H, Zhang J (2015) Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour Technol 180:32–39
Frengova, G. I. & Beshkova, D. M. (2009) Carotenoids from Rhodotorula and Phaffiya: yeasts of biotechnological importance. 163–180. doi:https://doi.org/10.1007/s10295-008-0492-9
Osorio-González C, Hegde K, Brar S, Kermanshahipour A, Avalos Ramirez A (2018) Challenges in lipid production from lignocellulosic biomass using Rhodosporidium sp.; a look at the role of lignocellulosic inhibitors. Biofuels Bioprod Biorefining 13
Koutinas AA, Chatzifragkou A, Kopsahelis N, Papanikolaou S, Kookos IK (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 116:566–577
Karamerou EE, Webb C (2019) Cultivation modes for microbial oil production using oleaginous yeasts – a review. Biochem Eng J 151:107322
Yuzbasheva EY, Mostova EB, Andreeva NI, Yuzbashev TV, Laptev IA, Sobolevskaya TI, Sineoky SP (2017) Co-expression of glucose-6-phosphate dehydrogenase and acyl-CoA binding protein enhances lipid accumulation in the yeast Yarrowia lipolytica. New Biotechnol 39:18–21
Dong T, Knoshaug EP, Pienkos PT, Laurens LML (2016) Lipid recovery from wet oleaginous microbial biomass for biofuel production: a critical review. Appl Energy 177:879–895
Pawar PP, Vadgama RN, Lali AM, Odaneth (2020) Extractive production of microbial oil using hydrophobic adsorbents: a comparative study. Eng Rep 2:1–15
Warke MA, Pawar PP, Kothari SD, Odaneth AA, Lali AM. (2017) Two stage approach for microbial oil production using Yarrowia lipolytica NCIM 3590. Adv Biotech & Micro.; 5(1): 555652. DOI: 10.19080/AIBM.2017.05.555652
Yao L, Lee S-L, Wang T, Gerde (2013) Comparison of lipid extraction from microalgae and soybeans with aqueous isopropanol. J Am Oil Chem Soc 90:571–578
Park P, Kim E, Chu (2007) Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep Purif Technol - SEP PURIF TECHNOL 53:148–152
Kanzy HM, Nasr NF, El-shazly HAM, Barakat (2015) Optimization of carotenoids production by yeast strains of Rhodotorula using salted cheese whey. Int J Curr Microbiol App Sci 4:456–469
Zeppa G, Conterno L, Gerbi (2001) Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J Agric Food Chem 49:2722–2726
Zhang X, Liu M, Zhang X, Tan (2018) Microbial lipid production and organic matters removal from cellulosic ethanol wastewater through coupling oleaginous yeasts and activated sludge biological method. Bioresour Technol 267:395–400
Shantha NC, Napolitano (1992) Gas chromatography of fatty acids. J Chromatogr 624:37–51
Patel A, Arora N, Mehtani J, Pruthi V, Pruthi (2017) Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew Sust Energ Rev 77:604–616
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Kumar D, Singh B, Korstad (2017) Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew Sust Energ Rev 73:654–671
Kahr H, Pointner M, Krennhuber K, Wallner B, Jaeger (2015) Lipid production from diverse oleaginous yeasts from steam exploded corn cobs. Agron Res 13:318–327
Chaiyaso T, Techapun C, Watanabe M, Manowattana (2019) Efficient bioconversion of enzymatic corncob hydrolysate into biomass and lipids by oleaginous yeast Rhodosporidium paludigenum KM281510. Prep Biochem Biotechnol 49:545–556
Pawar PP, Odaneth AA, Vadgama RN, Lali AM (2019) Simultaneous lipid biosynthesis and recovery for oleaginous yeast Yarrowia lipolytica. Biotechnol Biofuels 12:237
Poontawee R, Yongmanitchai W, Limtong S (2017) Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem 53:44–60
Lorenz E, Runge D, Marbà-Ardébol AM, Schmacht M, Stahl U, Senz M (2017) Systematic development of a two-stage fed-batch process for lipid accumulation in Rhodotorula glutinis. J Biotechnol 246:4–15
Hu C, Zhao X, Zhao J, Wu S, Zhao (2009) Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. Bioresour Technol 100:4843–4847
Patel A, Arora N, Sartaj K, Pruthi V, Pruthi (2016) Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew Sust Energ Rev 62:836–855
Chen X, Huang C, Xiong L, Chen X, Ma L (2012) Microbial oil production from corncob acid hydrolysate by Trichosporon cutaneum. Biotechnol Lett 34:1025–1028
Hu C, Wu S, Wang Q, Jin G, Shen H, Zhao ZK (2011) Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum. Biotechnol Biofuels 4:25
Qi G-X, Huang C, Chen XF, Xiong L, Wang C, Lin XQ, Shi SL, Yang D, Chen XD (2016) Semi-pilot scale microbial oil production by Trichosporon cutaneum using medium containing corncob acid hydrolysate. Appl Biochem Biotechnol 179:625–632
Galafassi S, Cucchetti D, Pizza F, Franzosi G, Bianchi D, Compagno C (2012) Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour Technol 111:398–403
Ledesma-amaro R, Dulermo R, Niehus X, Nicaud J (2016) Combining metabolic engineering and process optimization to improve production and secretion of fatty acids. Metab Eng 38:38–46
Hamam S (1987) Diffusion of crude oil in water. J. Environ. Sci. Heal. Part A-toxic\/hazardous Subst. Environ Eng 22:445–456
Phillips T, Chase M, Wagner S, Renzi C, Powell M, DeAngelo J, Michels P (2013) Use of in situ solid-phase adsorption in microbial natural product fermentation development. J Ind Microbiol Biotechnol 40:411–425
Zinjarde SS, Pant A, Deshpande (1998) Dimorphic transition in Yarrowia lipolytica isolated from oil-polluted sea water. Mycol Res 102:553–558
Nojima Y, Yagi T, Miyakawa T, Matsuzaki H, Hatano T, Fukui S (1995) Extracellular formation of triglycerides from glucose by a mutant strain of Trichosporon. J Ferment Bioeng 80:88–90
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16:143–169
Yu X, Zheng Y, Dorgan KM, Chen S (2011) Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour Technol 102:6134–6140. https://doi.org/10.1016/j.biortech.2011.02.081
Patel A, Pruthi V, Singh RP, Pruthi PA (2015) Synergistic effect of fermentable and non-fermentable carbon sources enhances TAG accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour Technol 188:136–144. https://doi.org/10.1016/j.biortech.2015.02.062
Acknowledgements
The authors acknowledge the Institute of Chemical Technology, Mumbai (formerly U.D.C.T., Mumbai), and DBT-ICT Centre for Energy Biosciences for providing infrastructure for carrying this research work.
Funding
DVR and PPP gratefully acknowledge the Department of Biotechnology, Government of India, and DBT-ICT Centre for Energy Biosciences, Mumbai, for their fellowships. The authors are thankful to the Department of Biotechnology, Government of India, for financial support (Project grant No.: BT/EB/ICT-Extension/2012, 05/06/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 165 kb)
Rights and permissions
About this article
Cite this article
Rane, D.V., Pawar, P.P., Odaneth, A.A. et al. Microbial oil production by the oleaginous red yeast, Rhodotorula glutinis NCIM 3168, using corncob hydrolysate. Biomass Conv. Bioref. 13, 1987–1997 (2023). https://doi.org/10.1007/s13399-021-01298-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01298-z