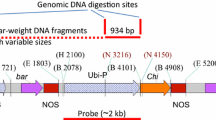
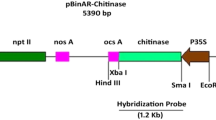
Abstract
A chitinase gene from rice (Rchit) was introduced into three varieties of peanut through Agrobacterium-mediated genetic transformation resulting in 30 transgenic events harboring the Rchit gene. Stable integration and expression of the transgenes were confirmed using PCR, RT-PCR and Southern blot analysis. Progeny derived from selfing of the primary transgenic events revealed a Mendelian inheritance pattern (3:1) for the transgenes. The chitinase activity in the leaves of the transgenic events was 2 to 14-fold greater than that in the non-transformed control plants. Seeds of most transgenic events showed 0–10 % A. flavus infection during in vitro seed inoculation bioassays. Transgenic peanut plants evaluated for resistance against late leaf spot (LLS) and rust using detached leaf assays showed longer incubation, latent period and lower infection frequencies when compared to their non-transformed counterparts. A significant negative correlation existed between the chitinase activity and the frequency of infection to the three tested pathogens. Three progenies from two transgenic events displayed significantly higher disease resistance for LLS, rust and A. flavus infection and are being advanced for further evaluations under confined field conditions to confirm as sources to develop peanut varieties with enhanced resistance to these fungal pathogens.







Similar content being viewed by others
Abbreviations
- LLS:
-
Late leaf spot
- SAT:
-
Semi arid tropics
- SEM:
-
Shoot elongation media
References
Anuradha TS, Divya K, Jami SK, Kirti PB (2008) Transgenic tobacco and peanut plants expressing a mustard defensin show resistance to fungal pathogens. Plant Cell Rep 27:1777–1786
Badigannavar AM, Kale DM, Mondal S, Murty GSS (2005) Trombay groundnut recombinants resistant to foliar diseases. Mutat Breed Newsl Rev 1:11–12
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carstens M, Vivier MA, Pretorius IS (2003) The Saccharomyces cerevisiae chitinase, encoded by the CTS1-2 gene, confers antifungal activity against Botrytis cinerea to transgenic tobacco. Transg Res 12:497–508
Datta K, Koukolikova-Nicola Z, Baisakh N, Oliva N, Datta SK (2000) Agrobacterium-mediated engineering for sheath blight resistance of indica rice cultivars from different ecosystems. Theor Appl Genet 100:832–839
Datta K, Tu J, Oliva N, Ona I, Velazhahan R, Mew TW, Muthukrishnan S, Datta SK (2001) Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci 160:405–414
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 4:19–21
Emani C, Garcia JM, Lopata-Finch E, Pozo MJ, Uribe P, Kim DJ, Sunilkumar G, Cook DR, Kenerley CM, Rathore KS (2003) Enhanced fungal resistance in transgenic cotton expressing an endochitinase gene from Trichoderma virens. Plant Biotechnol J 1:321–336
Ganesan M, Bhanumathi P, Ganesh Kumari K, Lakshmi Prabha A, Pill-Soon S, Jayabalan N (2009) Transgenic Indian Cotton (Gossypium hirsutum) harboring rice chitinase gene (Chi II) confers resistance to two fungal pathogens. Am J Biochem Biotechnol 5:63–74
Grison R, Grezes-Basset B, Schneider M, Lucante N, Olsen L, Leguay JJ, Toppan A (1996) Field tolerance to fungal pathogens of Brassica napus constitutively expressing a chimeric chitinase gene. Nat Biotechnol 14:643–646
Hossain MD, Rahman MZ, Abeda K, Rahman MM (2007) Screening of groundnut genotypes for leaf spots and rust resistance. Int J Sustain Crop Prod 2:7–10
Huang JK, Wen L, Swegle M, Tran HC, Thin TH, Naylor HM, Muthukrishnan S, Reeck GR (1991) Nucleotide sequence of a rice genomic clone that encodes a class I endochitinase. Plant Mol Biol 16:479–480
Iseli B, Boller T, Neuhaus JM (1993) The N-terminal cysteine rich domain of tobacco class I chitinase is essential for chitin binding but not for catalytic or antifungal activity. Plant Physiol 103:221–226
Itoh Y, Takahashi K, Takizawa H, Nikaidou N, Tanaka H, Nishihashi H, Watanabe T, Nishizawa Y (2003) Family 19 chitinase of Streptomyces griseus HUT6037 increases plant resistance to the fungal disease. Biosci Biotechnol Biochem 67:847–855
Jwanny EW, El-Sayed ST, Salem AM, Shehata AN (2001) Characterization and antifungal evaluation of chitinase and laminarinases from sugarbeet leaves. Pak J Biol Sci 4:271–276
Kishimoto K, Nishizawa Y, Tabei Y, Hibi T, Nakajima M, Akutsu K (2002) Detailed analysis of rice chitinase gene expression in transgenic cucumber plants showing different levels of disease resistance to gray mold (Botrytis cinerea). Plant Sci 162:655–662
Liang XQ, Guo BZ, Holbrook CC, Lynch RE (1994) Resistance to Aspergillus flavus in peanut seeds is associated with constitutive trypsin inhibitor and inducible chitinase and β-1,3-glucanase. Proc Amer Peanut Res Edu Soc, July 8–11, 2003, Clear Water, Florida 35:85
Lin W, Anuratha CS, Datta K, Potrykus I, Muthukrishnan S, Datta SK (1995) Genetic engineering of rice for resistance to sheath blight. Nat Biotechnol 13:686–691
Livingstone DM, Hampton JL, Phipps PM, Elizabeth AG (2005) Enhancing resistance to Sclerotinia minor in peanut by expressing a barley oxalate oxidase gene. Plant Physiol 137:1354–1362
Mauch F, Hadwiger LA, Boller T (1984) Ethylene: symptom, not signal for the induction of chitinase and β-1,3glucanase in pea pods by pathogens and elicitors. Plant Physiol 76:607–611
McDonald D, Subrahmanyam P, Gibbons RW, Smith DH (1985) Early and late leaf spots of groundnut. Information Bull No 21, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Andhra Pradesh, 502324, India, p 24
Moore KG, Price MS, Boston RS, Weissinger AK, Payne GA (2004) A chitinase from Tex6 maize kernels inhibits growth of Aspergillus flavus. Phytopathol 94:82–87
Nandakumar R, Babu S, Kalpana K, Raguchander T, Balasubramanian P, Samiyappan R (2007) Agrobacterium-mediated transformation of indica rice with chitinase gene for enhanced sheath blight resistance. Biol Plant 51:142–148
Pasonen HL, Seppanen SK, Degefu Y, Rytkonen A, Weissenberg K, Pappinen A (2004) Field performance of chitinase transgenic silver birches (Betula pendula): resistance to fungal diseases. Theor Appl Genet 109:562–570
Pensuk V, Patanothai A, Jogloy S, Wongkaew S, Akkasaeng C, Vorasoot N (2003) Reaction of peanut cultivars to late leafspot and rust. Songklanakarin J Sci Technol 25:289–295
Rao MJV, Upadhyaya HD, Mehan VK, Nigam SN, McDonald D, Reddy NS (1995) Registration of peanut germplasm ICGV 88145 and ICGV 89104 resistant to seed infection by Aspergillus flavus. Crop Sci 35:1717
Reddy LJ, Nigam SN, Subrahmanyam P, Reddy AGS, McDonald D, Gibbons RW, Pentaiah V (1992) Registration of ‘ICGV 87160’ peanut cultivar. Crop Sci 32:1075
Reddy LJ, Nigam SN, Moss JP, Singh AK, Subrahmanyam P, McDonald D, Reddy AGS (1996) Registration of ICGV 86699 peanut germplasm line with multiple disease and insect resistance. Crop Sci 36:821
Rohini VK, Rao KS (2001) Transformation of peanut (Arachis hypogaea L.) with tobacco chitinase gene: variable response of transformants to leaf spot disease. Plant Sci 160:889–898
Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, Van Den Elzen PJM, Cornelissen BJC (1993) Only specific tobacco (Nicotiana tobacum) chitinases and β-1,3-glucanases exhibit antifungal activity. Plant Physiol 101:857–863
Sharma KK, Anjaiah V (2000) An efficient method for the production of transgenic plants of peanut (Arachis hypogaea L.) through Agrobacterium tumefaciens mediated genetic transformation. Plant Sci 159:7–19
Sharma KK, Bhatnagar-Mathur P (2006) Peanut (Arachis hypogaea L.). In: Wang K (ed) Methods in molecular biology, Agrobacterium Protocols, vol 343. Springer, New Jersey, pp 347–358, ISBN 1-58829-536-2
Sharma KK, Bhatnagar-Mathur P, Saivishnupriya K, Anjaiah V, Vadez V, Waliyar F, Lava Kumar P, Nigam SN, Hoisington DH (2006) Genetic engineering of groundnut for crop improvement, 44 pp. In: Groundnut Aflatoxin Management and Genomics. Program and Book of Abstract. International Conf., 5–9 Nov. 2006, Guangzhou, Gungdong, China. Crops Research Institute, Guangdong Acad Agri Sci
Singh AK, Mehan VK, Nigam SN (1997) Sources of resistance to groundnut fungal and bacterial diseases: an update and appraisal. Information Bull No. 50. ICRISAT, Patancheru, p 48
Sreeramanan S, Maziah M, Xavier R (2009) A protocol for Agrobacterium-mediated transformation of banana with a rice chitinase gene. Emir J Food Agric 21:18–33
Subramanyam P, Rao VR, McDonald D, Moss JP, Gibbons RW (1989) Origins of resistance to rust and late leaf spot in peanut (Arachis hypogaea L.). Econ Bot 43:444–455
Sundaresha S, Manoj Kumar A, Rohini S, Math SA, Keshamma E, Chandrashekar SC, Udayakumar M (2009) Enhanced protection against two major fungal pathogens of groundnut, Cercospora arachidicola and Aspergillus flavus in transgenic groundnut over-expressing a tobacco β 1–3 glucanase. Eur J Plant Pathol 126:497–508
Tabei Y, Kitade S, Nishizawa Y, Kikuchi N, Kayano T, Hibi T, Akutsu K (1998) Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resistance to gray mold (Botrytis cinerea). Plant Cell Rep 17:159–164
Takahashi W, Fujimori M, Miura Y, Komatsu T, Nishizawa Y, Hibi T, Takamizo T (2005) Increased resistance to crown rust disease in transgenic Italian ryegrass (Lolium multiflorum Lam.) expressing the rice chitinase gene. Plant Cell Rep 23:811–818
Thakur RP, Rao VP, Reddy SV, Ferguson M (2000) Evaluation of wild Arachis germplasm accessions for in vitro seed colonization and aflatoxin production by Aspergillus flavus. Int Arachis Newsl 20:44–46
Waliyar F, Bosc JP, Bonkoungou S (1993) Sources of resistance to foliar diseases of groundnut and their stability in West Africa. Oleagineux 48:283–287
Waliyar F, Ba A, Hassan H, Bonkoungou S, Bosc JP (1994) Sources of resistance to Aspergillus flavus and aflatoxin contamination in groundnut genotypes in West Africa. Plant Dis 78:704–708
Waliyar F, Adomou M, Benin C, Traore A (2000) Rational use of fungicide applications to maximize peanut yield under foliar disease pressure in West Africa. Plant Dis 84:1203–1211
Yamamoto T, Iketani H, Ieki H, Nishizawa Y, Notsuka K, Hibi T, Hayashi T, Matsuta N (2000) Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep 19:639–646
Zhu H, Muthukrishnan S, Krishnaveni S, Wilde G, Jeoung JM, Liang GH (1998) Biolistic transformation of sorghum using a rice chitinase gene. J Genet Breed 52:243–252
Acknowledgments
We thank Dr. S. Muthukrishnan, Department of Biochemistry, Kansas State University, for kindly providing the rice chitinase gene. We also thank R. Kanaka Reddy for excellent technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasad, K., Bhatnagar-Mathur, P., Waliyar, F. et al. Overexpression of a chitinase gene in transgenic peanut confers enhanced resistance to major soil borne and foliar fungal pathogens. J. Plant Biochem. Biotechnol. 22, 222–233 (2013). https://doi.org/10.1007/s13562-012-0155-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-012-0155-9