Abstract
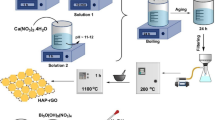
Manganese oxide-poly vinyl chloride (MnO2-PVC) composite has been synthesized for the removal of methylene blue from aqueous solution. Manganese oxide (MnO2) was first prepared by mixing manganese chloride tetra hydrated with tetramethylammonium hydroxide and hydrogen peroxide followed by functionalization with polyvinyl chloride (PVC). The successful synthesis of the composite was confirmed by Fourier transform infrared, X-ray diffraction and energy-dispersive X-ray analysis. The point of zero charge and the surface area of MnO2 nanosheets were increased from 4.10 and 214 m2/g to 5.01 and 226 m2g, respectively, after functionalization with PVC. The adsorption experiments under different experimental conditions, such as pH, concentration, time, dosage and temperature, were conducted to study the removal of methylene blue (MB) from aqueous solution by MnO2 and MnO2-PVC composite. The adsorption capacity of MnO2-PVC was 16 times greater than pristine MnO2, which is attributed to its high surface area, stability, polarizability and porosity. The Langmuir isotherm model was a good choice to probe into the mechanism of adsorption. Moreover, the adsorption process followed the pseudo-second-order kinetics, and the intra-particle diffusion was accompanied by the film diffusion for controlling the rate of adsorption. The thermodynamic parameters such as free energy, enthalpy and entropy indicated that the adsorption process was found to be exothermic and more spontaneous at 298 K. These results demonstrate that MnO2-PVC is an efficient, environment-friendly and versatile adsorbent for dyes removal from wastewater.






Similar content being viewed by others
References
Abdel Salam M (2015) Synthesis and characterization of novel manganese oxide nanocorals and their application for the removal of methylene blue from aqueous solution. Chem Eng J 270:50–57. https://doi.org/10.1016/j.cej.2015.02.022
Abdolmaleki A, Mallakpour S, Tabebordbar H (2017) Improvement of PVC/α-MnO2-LVA nanocomposites properties: a promising adsorbent for Pb(II) uptake. Int J Polym Anal Charact 5341:1–20
Aksu Z, Tatlı AI, Tunç Ö (2008) A comparative adsorption/biosorption study of acid blue 161: effect of temperature on equilibrium and kinetic parameters. Chem Eng J 142(1):23–39
Ali HMAMMS et al (2017) Chemically modified polyvinyl chloride for removal of thionine dye (Lauth’s violet). Materials 10(11):1–22. https://doi.org/10.3390/ma10111298
Arami M et al (2005) Removal of dyes from colored textile wastewater by orange peel adsorbent: equilibrium and kinetic studies. J Colloid Interface Sci 288:371–376. https://doi.org/10.1016/j.jcis.2005.03.020
Argun GOLGH (2020) Detoxification of waste hand paper towel hydrolysate by activated carbon adsorption. Int J Environ Sci Technol 17:799–808. https://doi.org/10.1007/s13762-019-02499-w
Azizullah A et al (2011) Water pollution in Pakistan and its impact on public health—a review. Environ Int 37(2):479–497. https://doi.org/10.1016/j.envint.2010.10.007
Banazadeh A et al (2016) Highly efficient degradation of hazardous dyes in aqueous phase by supported palladium nanocatalyst—a green approach. J Environ Chem Eng 4(2):2178–2186. https://doi.org/10.1016/j.jece.2015.09.007
Bao X et al (2018) In-situ generation of gold nanoparticles on MnO2 nanosheets for the enhanced oxidative degradation of basic dye (Methylene Blue). J Environ Sci (China) 65:236–245. https://doi.org/10.1016/j.jes.2017.03.003
Bharali D, Deka RC (2017) Preferential adsorption of various anionic and cationic dyes from aqueous solution over ternary CuMgAl layered double hydroxide. Colloids Surf A 525(February):64–76. https://doi.org/10.1016/j.colsurfa.2017.04.060
Borah D et al (2008) Surface-modified carbon black for As(V) removal. J Colloid Interface Sci 319:53–62
Carolin CF et al (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5:2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Chia CH et al (2013) Methylene blue adsorption on graphene oxide. Sains Malaysiana 42(6):819–826
Deng H et al (2019) Synthesis of fibrous LaFeO3 perovskite oxide for adsorption of Rhodamine B. Ecotoxicol Environ Saf 168:35–44. https://doi.org/10.1016/j.ecoenv.2018.09.056
Doǧan M, Alkan M (2003) Adsorption kinetics of methyl violet onto perlite. Chemosphere 50:517–528. https://doi.org/10.1016/S0045-6535(02)00629-X
El Qada EN, Allen SJ, Walker GM (2008) Adsorption of basic dyes from aqueous solution onto activated carbons. Chem Eng J 135(3):174–184. https://doi.org/10.1016/j.cej.2007.02.023
Fan X, Parker DJ, Smith MD (2003) Adsorption kinetics of fluoride on low cost materials. Water Res 37:4929–4937. https://doi.org/10.1016/j.watres.2003.08.014
Ferreira AM et al (2014) Complete removal of textile dyes from aqueous media using ionic-liquid-based aqueous two-phase systems. Sep Purif Technol 128:58–66. https://doi.org/10.1016/j.seppur.2014.02.036
Fida H et al (2017) Heterogeneous Fenton degradation of organic dyes in batch and fixed bed using La-Fe montmorillonite as catalyst. J Colloid Interface Sci 490:859–868. https://doi.org/10.1016/j.jcis.2016.11.085
Fontana KB et al (2016) Textile dye removal from aqueous solutions by malt bagasse: isotherm, kinetic and thermodynamic studies. Ecotoxicol Environ Saf 124:329–336. https://doi.org/10.1016/j.ecoenv.2015.11.012
Fu C et al (2020) The single/co-adsorption characteristics and microscopic adsorption mechanism of biochar-montmorillonite composite adsorbent for pharmaceutical emerging organic contaminant atenolol and lead ions. Ecotoxicol Environ Saf 187:109763. https://doi.org/10.1016/j.ecoenv.2019.109763
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Hua M et al (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211–212:317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016
Jamal R, Zhang L, Wang M, Zhao Q, Abdiryim T (2016) Synthesis of poly(3,4-propylenedioxythiophene)/MnO2 composites and their applications in the adsorptive removal of methylene blue. Prog Nat Sci Mater Int 26(1):32–40
Jorfi S, Shooshtarian MR, Pourfadakari S (2020) Decontamination of cadmium from aqueous solutions using zeolite decorated by Fe3O4 nanoparticles: adsorption modeling and thermodynamic studies. Int J Environ Sci Technol 17(1):273–286
Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Biores Technol 76(1):63–65. https://doi.org/10.1016/S0960-8524(00)00072-9
Kumar PS et al (2012) Kinetics, mechanism, isotherm and thermodynamic analysis of adsorption of cadmium ions by surface-modified Strychnos potatorum seeds. Korean J Chem Eng 29(12):1752–1760. https://doi.org/10.1007/s11814-012-0077-1
Kumar BMP et al (2014) Preparation of MnO 2 nanoparticles for the adsorption of environmentally hazardous malachite green dye. Int J Appl Innov Eng Manag 3(12):102–106
Li Y et al (2015) Synthesis of BiOBr-PVP hybrids with enhanced adsorption-photocatalytic properties. Appl Surf Sci 347:258–264
Liu S et al (2014) Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N,N-dimethyl dehydroabietylamine oxide. Chem Eng J 248:135–144. https://doi.org/10.1016/j.cej.2014.03.026
Madrakian T et al (2011) Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J Hazard Mater 196:109–114. https://doi.org/10.1016/j.jhazmat.2011.08.078
Mahmood T, Khan A, Naeem A, Hamayun M, Muska M, Farooq M, Hussain F (2015) Adsorption of Ni(II) ions from aqueous solution onto a fungus. Desalin Water Treat 57(16):7209–7218
Mallakpour S, Motirasoul F (2018) Ultrasonication synthesis of PVA/PVP/α-MnO 2 -stearic acid blend nanocomposites for adsorbing Cd II ion. Ultrason Sonochem 40:410–418
Mallakpour S, Abdolmaleki A, Tabebordbar H (2016) Production of PVC/α-MnO2-KH550 nanocomposite films: morphology, thermal, mechanical and Pb(II) adsorption properties. Eur Polym J 78:141–152. https://doi.org/10.1016/j.eurpolymj.2016.03.022
Maruthapandi M, Kumar VB, Gedanken A (2018) Carbon dot initiated synthesis of poly(4,4′-diaminodiphenylmethane) and its methylene blue adsorption. ACS Omega 3(6):7061–7068
Ortega PFR et al (2017) Thermodynamic study of methylene blue adsorption on carbon nanotubes using isothermal titration calorimetry: a simple and rigorous approach. J Chem Eng Data 62(2):729–737. https://doi.org/10.1021/acs.jced.6b00804
Pang J et al (2017) Adsorption behaviors of methylene blue from aqueous solution on mesoporous birnessite. J Taiwan Inst Chem Eng 77:168–176. https://doi.org/10.1016/j.jtice.2017.04.041
Peng L et al (2017) Highly efficient removal of methylene blue from aqueous solution by a novel fishing-net effect of manganese oxide nano-sheets. Clean Technol Environ Policy 19(1):269–277. https://doi.org/10.1007/s10098-016-1214-z
Rath SS et al (2017) Adsorption of heavy metals on a complex Al-Si-O bearing mineral system: insights from theory and experiments. Sep Purif Technol 186:28–38. https://doi.org/10.1016/j.seppur.2017.05.052
Ravi V et al (2006) Decolorization of distillery effluent using poly(vinyl chloride) and cellulose acetate phthalate as adsorbents. J Macromol Sci A Pure Appl Chem 43:1247–1254. https://doi.org/10.1080/10601320600737591
Sabna V, Thampi SG, Chandrakaran S (2016) Adsorption of crystal violet onto functionalized multi-walled carbon nanotubes: equilibrium and kinetic studies. Ecotoxicol Environ Saf 134:390–397. https://doi.org/10.1016/j.ecoenv.2015.09.018
Saleh TA, Gupta VK (2012) Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ Sci Pollut Res 19:1224–1228. https://doi.org/10.1007/s11356-011-0670-6
Soltani-nezhad F et al (2020) Synthesis of Fe3O4/CdS–ZnS nanostructure and its application for photocatalytic degradation of chlorpyrifos pesticide and brilliant green dye from aqueous solutions. Ecotoxicol Environ Saf 189:109886. https://doi.org/10.1016/j.ecoenv.2019.109886
Tang Y et al (2019) Ultrafast and efficient removal of anionic dyes from wastewater by polyethyleneimine-modified silica nanoparticles. Chemosphere 229:570–579. https://doi.org/10.1016/j.chemosphere.2019.05.062
Tran HV, Bui LT, Dinh TT, Le DH, Huynh CD, Trinh AX (2017) Graphene oxide/Fe3O4 /chitosan nanocomposite: a recoverable and recyclable adsorbent for organic dyes removal. Application to methylene blue. Mater Res Express 4(3):035701
Uysal M, Ar I (2007) Removal of Cr(VI) from industrial wastewaters by adsorption. Part I: determination of optimum conditions. J Hazard Mater 149(2):482–491. https://doi.org/10.1016/j.jhazmat.2007.04.019
Wang F, Zhang L, Wang Y, Liu X, Rohani S, Lu J (2017) Fe3O4 @SiO2 @CS-TETA functionalized graphene oxide for the adsorption of methylene blue (MB) and Cu(II). Appl Surf Sci 420:970–981
Wan Ngah WS, Hanafiah MAKM (2008) ‘Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Biores Technol. https://doi.org/10.1016/j.biortech.2007.06.011
Yu L et al (2018) Pectin microgel particles as high adsorption rate material for methylene blue: performance, equilibrium, kinetic, mechanism and regeneration studies. Int J Biol Macromol 112:383–389. https://doi.org/10.1016/j.ijbiomac.2018.01.193
Zhang SW, Chen GZ (2009) Manganese oxide based materials for supercapacitors. Energy Mater 3:186–200. https://doi.org/10.1179/174892409X427940
Zhang C et al (2019) Synthesis and Zn(II) modification of hierarchical porous carbon materials from petroleum pitch for effective adsorption of organic dyes. Chemosphere 216:379–386. https://doi.org/10.1016/j.chemosphere.2018.10.164
Zhu W et al (2013) Rapid removal of Cr(VI) ions from aqueous solutions by the macroporous poly(N, N′-dimethylamino ethyl methacrylate) hydrogels. J Appl Polym Sci 128(5):2729–2735. https://doi.org/10.1002/app.38409
Acknowledgements
The authors would like to convey their gratefulness to the National Centre of Excellence in Physical Chemistry, University of Peshawar, for providing us the necessary support and facilities to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Agnieszka Galuszka.
Rights and permissions
About this article
Cite this article
Saeed, T., Naeem, A., Mahmood, T. et al. Comparative study for removal of cationic dye from aqueous solutions by manganese oxide and manganese oxide composite. Int. J. Environ. Sci. Technol. 18, 659–672 (2021). https://doi.org/10.1007/s13762-020-02844-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02844-4