Abstract
BACKGROUND:
Mesenchymal stromal cells (MSCs) are multipotent stem cells that can differentiate into several cell types. In addition, many studies have shown that MSCs modulate the immune response. However, little information is currently available regarding the maintenance of immunomodulatory characteristics of MSCs through passages. Therefore, we investigated and compared cytokine and gene expression levels from adipose (AD) and bone marrow (BM)-derived MSCs relevant to immune modulation from early to late passages.
METHODS:
MSC immunophenotype, growth characteristics, cytokine expressions, and gene expressions were analyzed.
RESULTS:
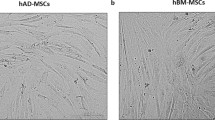
AD-MSCs and BM-MSCs had similar cell morphologies and surface marker expressions from passage 4 to passage 10. Cytokines secreted by AD-MSCs and BM-MSCs were similar from early to late passages. AD-MSCs and BM-MSCs showed similar immunomodulatory properties in terms of cytokine secretion levels. However, the gene expressions of tumor necrosis factor-stimulated gene (TSG)-6 and human leukocyte antigen (HLA)-G were decreased and gene expressions of galectin-1 and -3 were increased in both AD- and BM-MSCs with repeated passages.
CONCLUSION:
Our study showed that the immunophenotype and expression of immunomodulation-related cytokines of AD-MSCs and BM-MSCs immunomodulation through the passages were not significantly different, even though the gene expressions of both MSCs were different.



Similar content being viewed by others
Change history
12 January 2019
Unfortunately, the online published article has error in Figure?3. The name of protein, ?Gelectin-1? should be corrected to ?Galectin-3?. The corrected Fig.?3 is placed in the following page.
12 January 2019
Unfortunately, the online published article has error in Figure?3. The name of protein, ?Gelectin-1? should be corrected to ?Galectin-3?. The corrected Fig.?3 is placed in the following page.
References
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72.
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85.
Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7.
Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96.
Subbanna PK. Mesenchymal stem cells for treating GVHD: in vivo fate and optimal dose. Med Hypotheses. 2007;69:469–70.
Wagner W, Bork S, Lepperdinger G, Joussen S, Ma N, Strunk D, et al. How to track cellular aging of mesenchymal stromal cells? Aging (Albany NY). 2010;2:224–30.
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–97.
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301.
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–6.
Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11:166–74.
Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–25.
Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51–60.
Pawitan JA. Prospect of adipose tissue derived mesenchymal stem cells in regenerative medicine. Cell Tissue Transpl Ther. 2009;2:7–9.
Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–41.
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213.
Thejaswi K, Amarnath M, Srinivas G, Jerald MK, Avinash Raj T, Singh S. Immune modulatory responses of mesenchymal stem cells from different sources in cultures and in vivo. Cell Tissue Transpl Ther. 2012;4:1–13.
Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–32.
Varma TK, Toliver-Kinsky TE, Lin CY, Koutrouvelis AP, Nichols JE, Sherwood ER. Cellular mechanisms that cause suppressed gamma interferon secretion in endotoxin-tolerant mice. Infect Immun. 2001;69:5249–63.
Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–53.
Tsai TL, Li WJ. Identification of bone marrow-derived soluble factors regulating human mesenchymal stem cells for bone regeneration. Stem Cell Reports. 2017;8:387–400.
Teshima T, Matsumoto H, Koyama H. Soluble factors from adipose tissue-derived mesenchymal stem cells promote canine hepatocellular carcinoma cell proliferation and invasion. PLoS One. 2018;13:e0191539.
Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A. 2016;113:158–63.
Kapasi K, Albert SE, Yie S, Zavazava N, Librach CL. HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology. 2000;101:191–200.
Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–44.
Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology. 2010;131:118–27.
Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–9.
Wang J, Xia J, Zhang F, Shi Y, Wu Y, Pu H, et al. Galectin-1-secreting neural stem cells elicit long-term neuroprotection against ischemic brain injury. Sci Rep. 2015;5:9621.
Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71:267–74.
Tsai HF, Wu CS, Chen YL, Liao HJ, Chyuan IT, Hsu PN. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J Mol Med (Berl). 2016;94:545–56.
Acknowledgements
This study was supported by a Grant of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2058120). The authors are grateful to Dong-Su Jang, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
BM-MSCs were provided at passage 2 by the Cell Therapy Center, Severance Hospital (Yonsei University College of Medicine, Seoul, Korea) from three donors after taking their consent. AD-MSCs were obtained from two donors (32- and 41-year old females) undergoing plastic surgery, after written informed consent, in accordance with the Institutional Review Boards (4-2010-0236) of Severance Hospital and one donor from ThermoFisher Scientific (R7788115).
Rights and permissions
About this article
Cite this article
Mun, C.H., Kang, MI., Shin, Y.D. et al. The Expression of Immunomodulation-Related Cytokines and Genes of Adipose- and Bone Marrow-Derived Human Mesenchymal Stromal Cells from Early to Late Passages. Tissue Eng Regen Med 15, 771–779 (2018). https://doi.org/10.1007/s13770-018-0147-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-0147-5