Abstract
Background and Objective
Little is understood about neonatal pharmacokinetics immediately after delivery and during the first days of life following intrauterine exposure to maternal medications. Our objective was to develop and evaluate a novel, physiologically based pharmacokinetic modeling workflow for predicting perinatal and postnatal disposition of commonly used antiretroviral drugs administered prenatally to pregnant women living with human immunodeficiency virus.
Methods
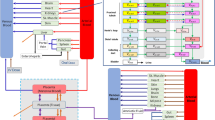
Using previously published, maternal-fetal, physiologically based pharmacokinetic models for emtricitabine, dolutegravir, and raltegravir built with PK-Sim/MoBi®, placental drug transfer was predicted in late pregnancy. The total drug amount in fetal compartments at term delivery was estimated and subsequently integrated as initial conditions in different tissues of a whole-body, neonatal, physiologically based pharmacokinetic model to predict drug concentrations in the neonatal elimination phase after birth. Neonatal elimination processes were parameterized according to published data. Model performance was assessed by clinical data.
Results
Neonatal physiologically based pharmacokinetic models generally captured the initial plasma concentrations after delivery but underestimated concentrations in the terminal phase. The mean percentage error for predicted plasma concentrations was − 71.5%, − 33.8%, and 76.7% for emtricitabine, dolutegravir, and raltegravir, respectively. A sensitivity analysis suggested that the activity of organic cation transporter 2 and uridine diphosphate glucuronosyltransferase 1A1 during the first postnatal days in term newborns is ~11% and ~30% of that in adults, respectively.
Conclusions
These findings demonstrate the general feasibility of applying physiologically based pharmacokinetic models to predict washout concentrations of transplacentally acquired drugs in newborns. These models can increase the understanding of pharmacokinetics during the first postnatal days and allow the prediction of drug exposure in this vulnerable population.


taken from an in vivo study of Hirt et al. [17], IMPAACT P1026 [10], and Clarke et al. [16]. a Emtricitabine 400-mg single dose in pregnant women with an average gestational age of 39 weeks at delivery. Empty circles represent individual concentration data in the maternal plasma taken from an in vivo study of Hirt et al. [17]; the line represents the predicted mean concentrations in the maternal plasma; the shaded area represents the predicted 5th–95th percentile range of the prediction. b Emtricitabine 400-mg single dose in pregnant women with an average gestational age of 39 weeks at delivery. Empty circles represent individual concentration data in the umbilical vein taken from an in vivo study of Hirt et al. [17] The line represents the predicted mean concentrations in the umbilical vein. The shaded area represents the predicted 5th–95th percentile range of the prediction. c Emtricitabine 400-mg single dose in pregnant women with an average gestational age of 39 weeks at delivery. The line represents the predicted mean amount of emtricitabine in the fetus. The marks represent the delivery time after the last dose. d Dolutegravir 50 mg once a day in pregnant women with an average gestational age of 38 weeks at delivery. Empty circles represent individual concentration data in the maternal plasma taken from an in vivo study of IMPAACT P1026; [10] the line represents the predicted mean concentration in the maternal plasma; the shaded area represents the predicted 5th–95th percentile range of the prediction. e Dolutegravir 50 mg once a day in pregnant women with an average gestational age of 38 weeks at delivery. Empty circles represent individual concentration data in the umbilical vein taken from an in vivo study of IMPAACT P1026; [10] the line represents the predicted mean concentration in the umbilical vein. The shaded area presents the predicted 5th–95th percentile range of the prediction. f Dolutegravir 50 mg once a day in pregnant women with an average gestational age of 38 weeks at delivery; the line represents the predicted mean amount of dolutegravir in the fetus. The marks represent the delivery time after the last dose. g Raltegravir 400 mg twice a day in pregnant women with an average gestational age of 38 weeks at delivery. Empty circles represent individual concentration data in the maternal plasma taken from an in vivo study of Clarke et al. [16]; the line represents the predicted mean concentrations in the maternal plasma; the shaded area represents the predicted 5th–95th percentile range of the prediction. h Raltegravir 400 mg twice a day in pregnant women with an average gestational age of 38 weeks at delivery. Empty circles represent individual concentration data in the umbilical vein taken from an in vivo study of Clarke et al. [16]; the line represents the predicted mean concentrations in the umbilical vein. The shaded area represents the predicted 5th–95th percentile range of the prediction. i Raltegravir 400 mg twice a day in pregnant women with an average gestational age of 38 weeks at delivery. The line represents the predicted mean amount of raltegravir in the fetus. The marks represent the delivery time after the last dose. conc concentration

taken from Hirt et al. [17]. b Dolutegravir plasma concentration in newborns; maternal dose of 50 mg once a day. Observed data were taken from IMPAACT P1026 [10]. c Raltegravir plasma concentration in newborns; maternal dose of 400 mg twice a day. Observed data were taken from Clarke et al. [16] conc concentration

taken from Hirt et al. [17] and black circles represent median observed data. b Emtricitabine plasma concentration in newborns with differing unbound fractions; maternal dose of 400 mg single dose. Empty circles represent observed data taken from Hirt et al. [17] and black circles represent median observed data. c Dolutegravir plasma concentration in newborns with differing uridine diphosphate glucuronosyltransferase 1A1 and cytochrome P450 3A4 activities; maternal dose of 50 mg once a day. Empty circles represent observed data taken from IMPAACT P1026 [10] and black circles represent median observed data. d Dolutegravir plasma concentration in newborns with differing unbound fractions; maternal dose of 50 mg once a day. Empty circles represent observed data taken from IMPAACT P1026 [10] and black circles represent median observed data. e Raltegravir plasma concentration in newborns with differing uridine diphosphate glucuronosyltransferase 1A1 activity and renal elimination; maternal dose of 400 mg twice a day. Empty circles represent observed data taken from Clarke et al. [16] and black circles represent median observed data. f Raltegravir plasma concentration in newborns with differing unbound fractions; maternal dose of 50 mg once a day. Empty circles represent observed data taken from Clarke et al. [16] and black circles represent median observed data. conc concentration

taken from Hirt et al. [17] and black circles represent median observed data. b Dolutegravir plasma concentration in newborns with differing dosing; maternal dose of 50 mg once a day. Empty circles represent observed data taken from IMPAACT P1026 [10] and black circles represent median observed data. c Raltegravir plasma concentration in newborns with differing dosing; maternal dose of 400 mg twice a day. Empty circles represent observed data taken from Clarke et al. [16] and black circles represent median observed data. conc concentration
Similar content being viewed by others
References
Pariente G, Leibson T, Carls A, Adams-Webber T, Ito S, Koren G. Pregnancy-associated changes in pharmacokinetics: a systematic review. PLoS Med. 2016;13(11):e1002160.
Wang J, Avant D, Green D, Seo S, Fisher J, Mulberg AE, et al. A survey of neonatal pharmacokinetic and pharmacodynamic studies in pediatric drug development. Clin Pharmacol Ther. 2015;98(3):328–35.
Van Den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl. 10):S10-25.
Ku LC, Smith PB. Dosing in neonates: special considerations in physiology and trial design. Pediatr Res. 2015;77(1–1):2–9.
Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21(39):5688–98.
Michelet R, Bocxlaer JV, Vermeulen A. PBPK in preterm and term neonates: a review. Curr Pharm Des. 2017;23(38):5943–54.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. Preterm physiologically based pharmacokinetic model. Part II: applications of the model to predict drug pharmacokinetics in the preterm population. Clin Pharmacokinet. 2020;59(4):501–18.
Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, et al. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13(4):226–35.
Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS. 2013;27(5):739–48.
Mulligan N, Best BM, Wang J, Capparelli EV, Stek A, Barr E, et al. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS. 2018;32(6):729–37.
Bollen P, Freriksen J, Konopnicki D, Weizsacker K, Hidalgo Tenorio C, Molto J, et al. The effect of pregnancy on the pharmacokinetics of total and unbound dolutegravir and its main metabolite in women living with human immunodeficiency virus. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa006.
Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014;67(4):375–81.
Blonk MI, Colbers AP, Hidalgo-Tenorio C, Kabeya K, Weizsacker K, Haberl AE, et al. Raltegravir in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis. 2015;61(5):809–16.
Liu XI, Momper JD, Rakhmanina N, Van Den Anker JN, Green DJ, Burckart GJ, et al. Physiologically based pharmacokinetic models to predict maternal pharmacokinetics and fetal exposure to emtricitabine and acyclovir. J Clin Pharmacol. 2020;60(2):240–55.
Liu XI, Momper JD, Rakhmanina NY, Green DJ, Burckart GJ, Cressey TR, et al. Prediction of maternal and fetal pharmacokinetics of dolutegravir and raltegravir using physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2020;59(11):1433–50.
Clarke DF, Acosta EP, Rizk ML, Bryson YJ, Spector SA, Mofenson LM, et al. Raltegravir pharmacokinetics in neonates following maternal dosing. J Acquir Immune Defic Syndr. 2014;67(3):310–5.
Hirt D, Urien S, Rey E, Arrive E, Ekouevi DK, Coffie P, et al. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53(3):1067–73.
Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: a randomised trial (DolPHIN-1 study). PLoS Med. 2019;16(9):e1002895.
Lippert J, Burghaus R, Edginton A, Frechen S, Karlsson M, Kovar A, et al. Open Systems Pharmacology community: an open access, open source, open science approach to modeling and simulation in pharmaceutical sciences. CPT Pharmacometrics Syst Pharmacol. 2019;8(12):878–82.
Dallmann A, Solodenko J, Ince I, Eissing T. Applied concepts in PBPK modeling: how to extend an Open Systems Pharmacology model to the special population of pregnant women. CPT Pharmacometrics Syst Pharmacol. 2018;7(7):419–31.
US Food and Drug Administration. Emtriva™ (emtricitabine) capsules. 2003. https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/21500_emtriva_lbl.pdf. Accessed 10 Jun 2020.
Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41(2):353–61.
Kassahun K, Mcintosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos. 2007;35(9):1657–63.
Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56(11):1303–30.
Dallmann A, Ince I, Solodenko J, Meyer M, Willmann S, Eissing T, et al. Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin Pharmacokinet. 2017;56(12):1525–41.
Reznicek J, Ceckova M, Cerveny L, Muller F, Staud F. Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica. 2017;47(1):77–85.
Cheung KWK, Van Groen BD, Spaans E, Van Borselen MD, De Bruijn A, Simons-Oosterhuis Y, et al. A comprehensive analysis of ontogeny of renal drug transporters: mRNA analyses, quantitative proteomics, and localization. Clin Pharmacol Ther. 2019;106(5):1083–92.
Badee J, Qiu N, Collier AC, Takahashi RH, Forrest WF, Parrott N, et al. Characterization of the ontogeny of hepatic UDP-glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. J Clin Pharmacol. 2019;59(Suppl. 1):S42-55.
Acharya C, Hooker AC, Turkyilmaz GY, Jonsson S, Karlsson MO. A diagnostic tool for population models using non-compartmental analysis: the ncappc package for R. Comput Methods Programs Biomed. 2016;127:83–93.
Codaccioni M, Brochot C. Assessing the impacts on fetal dosimetry of the modelling of the placental transfers of xenobiotics in a pregnancy physiologically based pharmacokinetic model. Toxicol Appl Pharmacol. 2020;409:115318.
Lommerse J, Clarke D, Kerbusch T, Merdjan H, Witjes H, Teppler H, et al. Maternal-neonatal raltegravir population pharmacokinetics modeling: implications for initial neonatal dosing. CPT Pharmacometrics Syst Pharmacol. 2019;8(9):643–53.
Yamamoto K, Fukushima S, Mishima Y, Hashimoto M, Yamakawa K, Fujioka K, et al. Pharmacokinetic assessment of alprazolam-induced neonatal abstinence syndrome using physiologically based pharmacokinetic model. Drug Metab Pharmacokinet. 2019;34(6):400–2.
Li H, Lampe JN. Neonatal cytochrome P450 CYP3A7: a comprehensive review of its role in development, disease, and xenobiotic metabolism. Arch Biochem Biophys. 2019;673:108078.
Miyagi SJ, Milne AM, Coughtrie MW, Collier AC. Neonatal development of hepatic UGT1A9: implications of pediatric pharmacokinetics. Drug Metab Dispos. 2012;40(7):1321–7.
Moss DM, Kwan WS, Liptrott NJ, Smith DL, Siccardi M, Khoo SH, et al. Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob Agents Chemother. 2011;55(2):879–87.
Bunglawala F, Rajoli RKR, Mirochnick M, Owen A, Siccardi M. Prediction of dolutegravir pharmacokinetics and dose optimization in neonates via physiologically based pharmacokinetic (PBPK) modelling. J Antimicrob Chemother. 2020;75(3):640–7.
Ota Y, Maruo Y, Matsui K, Mimura Y, Sato H, Takeuchi Y. Inhibitory effect of 5beta-pregnane-3alpha,20beta-diol on transcriptional activity and enzyme activity of human bilirubin UDP-glucuronosyltransferase. Pediatr Res. 2011;70(5):453–7.
Shibuya A, Itoh T, Tukey RH, Fujiwara R. Impact of fatty acids on human UDP-glucuronosyltransferase 1A1 activity and its expression in neonatal hyperbilirubinemia. Sci Rep. 2013;3:2903.
Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, et al. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother. 2012;56(6):3020–6.
US Food and Drug Administration. Clinical pharmacology review: dolutegravir, GSK1349572. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204790Orig1s000ClinPharmR.pdf. Accessed 29 Jun 2020.
Laufer R, Paz OG, Di Marco A, Bonelli F, Monteagudo E, Summa V, et al. Quantitative prediction of human clearance guiding the development of Raltegravir (MK-0518, isentress) and related HIV integrase inhibitors. Drug Metab Dispos. 2009;37(4):873–83.
Moss DM, Siccardi M, Back DJ, Owen A. Predicting intestinal absorption of raltegravir using a population-based ADME simulation. J Antimicrob Chemother. 2013;68(7):1627–34.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The NIH awards numbers 5T32HD087969-03 and 5T32HD087969-02 also support this project.
Conflicts of interest/Competing Interests
Xiaomei I. Liu, Jeremiah D. Momper, Natella Y. Rakhmanina, Dionna J. Green, Gilbert J. Burckart, Tim R. Cressey, Mark Mirochnick, Brookie M. Best, John N. van den Anker, and André Dallmann have no conflicts of interest with respect to the research, authorship, and/or publication of this article. André Dallmann is an employee of Bayer AG, a company that is part of the Open Systems Pharmacology member team and involved in the Open Systems Pharmacology software development used in this study. The results from this study will be presented in part at the American College of Clinical Pharmacology Annual Meeting, September 2020.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Not applicable.
Rights and permissions
About this article
Cite this article
Liu, X.I., Momper, J.D., Rakhmanina, N.Y. et al. Physiologically Based Pharmacokinetic Modeling Framework to Predict Neonatal Pharmacokinetics of Transplacentally Acquired Emtricitabine, Dolutegravir, and Raltegravir. Clin Pharmacokinet 60, 795–809 (2021). https://doi.org/10.1007/s40262-020-00977-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00977-w