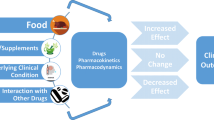
Abstract
Edoxaban, a factor Xa inhibitor, is a direct oral anticoagulant (DOAC). DOACs may have a lower incidence of drug-drug interactions (DDIs) than vitamin K antagonists such as warfarin. Nonetheless, DOACs can have clinically relevant pharmacokinetic and pharmacodynamic DDIs. Careful consideration of potential DDIs is needed when prescribing edoxaban, and clinically relevant DDIs may be prevented by avoiding offending combinations, spacing out the timing of the drugs or by halving the dose of edoxaban where indicated.
Similar content being viewed by others
References
Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55–61.
Corsini A, Ferri N, Proietti M, et al. Edoxaban and the issue of drug-drug interactions: from pharmacology to clinical practice. Drugs. 2020;80(11):1065–83.
Lixiana (edoxaban) 15, 30 and 60 mg film-coated tablets: summary of product characteristics. Munich: Daiichi Sankyo Europe GmbH; 2020.
Aisenberg J, Chatterjee-Murphy P, Friedman Flack K, et al. Gastrointestinal bleeding with edoxaban versus warfarin: results from the ENGAGE AF-TIMI 48 trial (effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction). Circ Cardiovasc Qual Outcomes. 2018;11(5):e003998.
Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170(16):1433–41.
Mendell J, Zahir H, Matsushima N, et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13:331–42.
Savaysa (edoxaban) tablets, for oral use: US prescribing information. New Jersey: Daiichi Sankyo Inc; 2020.
Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–53.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–933.
Matsushima N, Lee F, Sato T, et al. Bioavailability and safety of the factor Xa inhibitor edoxaban and the effects of quinidine in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(4):358–66.
Mendell J, Lee F, Chen S, et al. The effects of the antiplatelet agents, aspirin and naproxen, on pharmacokinetics and pharmacodynamics of the anticoagulant edoxaban, a direct factor Xa inhibitor. J Cardiovasc Pharmacol. 2013;62(2):212–21.
Parasrampuria DA, Mendell J, Shi M, et al. Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol. 2016;82(6):1591–600.
Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34(3):170–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
E.S. Kim is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. Y.-A. Heo is a salaried employee of Adis International/Springer Nature, is an editor of Drugs & Therapy Perspectives, was not involved in any publishing decision for the manuscript, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Kim, E.S., Heo, YA. Consider clinically relevant drug interactions when prescribing edoxaban. Drugs Ther Perspect 37, 115–119 (2021). https://doi.org/10.1007/s40267-020-00805-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-020-00805-y