Abstract
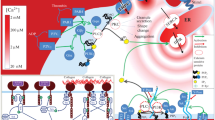
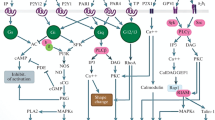
Platelets are nuclear-free cell fragments responsible for preventing blood loss in case of the vascular wall injury. After a contact with collagen exposed when the vessel is damaged, platelets become activated through the receptor glycoprotein GPVI. This leads to platelet shape change, degranulation, aggregation, and procoagulant responses. The aim of this study was to observe changes in the concentration of Ca2+ in the cytosol and functional responses of platelets upon stimulation through the GPVI receptor. The study involved healthy adult volunteers and house mice Mus musculus of the C57Bl6 line (wild type). Calcium signaling was monitored by Fura-Red fluorophore fluorescence using BD FACS Canto II flow cytometer. Functional responses were observed on a flow cytometer by binding of human fibrinogen conjugated to a fluorescent label or by Biola optical aggregometry. When tested on a cytometer, platelets were activated by collagen-related peptide CRP and when tested by aggregometry, platelets were activated using collagen. As a result of the study, the following phenomenon was revealed: despite a significant variability in the human platelet cytosolic Ca2+ levels in response to stimulation, the variability in activation of platelet integrins, shape change, and the aggregation response was significantly lower. No variability in the platelet cytosolic Ca2+ levels in response to stimulation was observed in mice. The observed variability of the calcium response of platelets could be caused by differences in the GPVI expression or polymorphisms of the GPVI receptor gene in the studied donors. Similarities in the functional responses of platelets with different signaling suggest a variable contribution of the phosphoinositide branch of intracellular signaling in platelets in response to activation of GPVI.




Similar content being viewed by others
REFERENCES
Weinberg C.B., Bell E. 1986. A blood vessel model constructed from collagen and cultured vascular cells. Science. 231 (4736), 397–400.
Monroe D.M., Hoffman M. 2006. What does it take to make the perfect clot? Arterioscler. Thromb. Vasc. Biol. 26 (1), 41–48.
Jackson S.P., Nesbitt W.S., Kulkarni S. 2003. Signaling events underlying thrombus formation. J. Thromb. Haemost. 1 (7), 1602–1612.
Siedlecki C.A., Lestini B.J., Kottke-Marchant K., Eppell S.J., Wilson D.L., Marchant R.E. 1996. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 88 (8), 2939–2950.
Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. 2016. Thrombosis and platelets: An update. Eur. Heart J. 38 (11), 785–791.
Yun S.H., Sim E.H., Goh R.Y., Park J.I., Han J.Y. 2016. Platelet activation: The mechanisms and potential biomarkers. Biomed Res. Int. 2016, 9060143.
Jackson S.P. 2011. Arterial thrombosis-insidious, unpredictable and deadly. Nature Medicine. 17 (11), 1423–1436.
Snell D.C., Schulte V., Jarvis G.E., Arase K., Sakurai D., Saito T., Watson S.P., Nieswandt B. 2002. Differential effects of reduced glycoprotein VI levels on activation of murine platelets by glycoprotein VI ligands. Biochem. J. 368 (1), 293–300.
Versteeg H.H., Heemskerk J.W.M., Levi M., Reitsma P.H. 2013. New fundamentals in hemostasis. Physiol. Rev. 93 (1), 327–358.
Bennett J.S. 2015. Regulation of integrins in platelets. Biopolymers. 104 (4), 323–333.
Lagrue-Lak-Hal A.H., Debili N., Kingbury G., Lecut C., Le Couedic J.P., Villeval J.L., Jandrot-Perrus M., Vainchenker W. 2001. Expression and function of the collagen receptor GPVI during megakaryocyte maturation. J. Biol. Chem. 276 (18), 15 316–15 325.
Nieswandt B., Watson S.P. 2003. Platelet-collagen interaction: Is GPVI the central receptor? Blood. 102 (2), 449–461.
Boylan B., Berndt M.C., Kahn M.L., Newman P.J. 2006. Activation-independent, antibody-mediated removal of GPVI from circulating human platelets: Development of a novel NOD/SCID mouse model to evaluate the in vivo effectiveness of anti-human platelet agents. Blood. 108 (3), 908–914.
Polgár J., Clemetson J.M., Kehrel B.E., Wiedemann M., Magnenat E.M., Wells T.N.C., Clemetson K.J. 1997. Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus (Tropical rattlesnake) venom via the p62/GPVI collagen receptor. J. Biol. Chem. 272 (21), 13 576–13 583.
Alshehri O.M., Hughes C.E., Montague S., Watson S.K., Frampton J., Bender M., Watson S.P. 2015. Fibrin activates GPVI in human and mouse platelets. Blood. 126 (13), 1601–1608.
Induruwa I., Moroi M., Bonna A., Malcor J.D., Howes J.M., Warburton E.A., Farndale R.W., Jung S.M. 2018. Platelet collagen receptor glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. J. Thromb. Haemost. 16 (2), 389–404.
Mangin P.H., Onselaer M.B., Receveur N., Le Lay N., Hardy A.T., Wilson C, Sanchez X., Loyau S., Dupuis A., Babar A.K., Miller J.L.C., Philippou H., Hughes C.E., Herr A.B., Ariëns R.A.S., Mezzano D., Jandrot-Perrus M., Gachet C., Watson S.P. 2018. Immobilized fibrinogen activates human platelets through glycoprotein VI. Haematologica. 103 (5), 898–907.
Getahun A., Cambier J.C. 2015. Of ITIMs, ITAMs, and ITAMis: Revisiting immunoglobulin Fc receptor signaling. Immunol. Rev. 268 (1), 66–73.
Andrews R.K., Suzuki-Inoue K., Shen Y., Tulasne D., Watson S.P., Berndt M.C. 2002. Interaction of calmodulin with the cytoplasmic domain of platelet glycoprotein VI. Blood. 99 (11), 4219–4221.
Moroi M., Jung S.M. 2004. Platelet glycoprotein VI: Its structure and function. Thromb. Res. 114 (4), 221–233.
Quek L., Pasquet J., Hers I., Cornall R., Knight G., Barnes M., Hibbs M., Dunn A., Lowell C., Watson S. 2001. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 96, 4246–4253.
Dumont B., Lasne D., Rothschild C., Bouabdelli M., Ollivier V., Oudin C., Ajzenberg N., Grandchamp B., Jandrot-Perrus M. 2009. Absence of collagen-induced platelet activation caused by compound heterozygous GPVI mutations. Blood. 114 (9), 1900–1903.
Hermans C., Wittevrongel C., Thys C., Smethurst P.A., Van Geet C., Freson K. 2009. A compound heterozygous mutation in glycoprotein VI in a patient with a bleeding disorder. J. Thromb. Haemost. 7 (8), 1356–1363.
Matus V., Valenzuela G., Sáez C.G., Hidalgo P., Lagos M., Aranda E., Panes O., Pereira J., Pillois X., Nurden A.T., Mezzano D. 2013. An adenine insertion in exon 6 of human GP6 generates a truncated protein associated with a bleeding disorder in four Chilean families. J. Thromb. Haemost. 11 (9), 1751–1759.
Arthur J.F., Dunkley S., Andrews R.K. 2007. Platelet glycoprotein VI-related clinical defects. British J. Haematology. 139 (3), 363–372.
Moroi M., Shinmyozut K., Hospital K.C. 1989. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J. Clin. Investig. 84, 1440–1445.
Nieswandt B., Bergmeier W., Schulte V., Rackebrandt K., Gessner J.E., Zirngibl H. (2000) Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRγ chain. J. Biol. Chem. 275 (31), 23 998–24 002.
Nieswandt B., Schulte V., Bergmeier W., Mokhtari-Nejad R., Rackebrandt K., Cazenave J.P., Ohlmann P., Gachet C., Zirngibl H. 2001. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J. Exp. Med. 193 (4), 459–469.
Dütting S., Bender M., Nieswandt B. 2012. Platelet GPVI: A target for antithrombotic therapy?! Trends Pharmacol. Sci. 33, 583–590.
Ungerer M., Rosport K., Bültmann A., Piechatzek R., Uhland K., Schlieper P., Gawaz M., Münch G. 2011. Novel antiplatelet drug revacept (dimeric glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation. 123 (17), 1891–1899.
Geahlen R.L. 2014. Getting Syk: Spleen tyrosine kinase as a therapeutic target. Trends Pharmacol. Sci. 35 (8), 414–422.
Mangin P.H., Tang C.J., Bourdon C., Loyau S., Freund M., Hechler B., Gachet C., Jandrot-Perrus M. 2012. A humanized glycoprotein VI (GPVI) mouse model to assess the antithrombotic efficacies of anti-GPVI agents. J. Pharmacol. Exp. Ther. 341 (1), 156–163.
Li H., Lockyer S., Concepcion A., Gong X., Takizawa H., Guertin M., Matsumoto Y., Kambayashi J., Tandon N.N., Liu Y. 2007. The fab fragment of a novel anti-GPVI monoclonal antibody, OM4, reduces in vivo thrombosis without bleeding risk in rats. Arterioscler. Thromb. Vasc. Biol. 27 (5), 1199–1205.
Takayama H., Hosaka Y., Nakayama K., Shirakawa K., Naitoh K., Matsusue T., Shinozaki M., Honda M., Yatagai Y., Kawahara T., Hirose J., Yokoyama T., Kurihara M., Furusako S. 2008. A novel antiplatelet antibody therapy that induces cAMP-dependent endocytosis of the GPVI/Fc receptor γ-chain complex. J. Clin. Investig. 118 (5), 1785–1795.
Morton L.F., Hargreaves P.G., Farndale R.W., Young R.D., Barnes M.J. 1995. Integrin α2β1-independent activation of platelets by simple collagen-like peptides: Collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for α2β1-independent platelet reactivity. Biochem. J. 306 (2), 337–344.
Cazenave J.P., Ohlmann P., Cassel D., Eckly A., Hechler B., Gachet C. 2004. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol. Biol. 272 (5), 13–28.
Alberio L., Ravanat C., Hechler B., Mangin P.H., Lanza F., Gachet C. 2017. Delayed-onset of procoagulant signalling revealed by kinetic analysis of COAT platelet formation. Thromb. Haemost. 117 (06), 1101–1114.
Martyanov A.A., Morozova D.S., Sorokina M.A., Filkova A.A., Fedorova D.V., Uzueva S.S., Suntsova E.V., Novichkova G.A., Zharkov P.A., Panteleev M.A., Sveshnikova A.N. 2020. Heterogeneity of integrin αIIbβ3 function in pediatric immune thrombocytopenia revealed by continuous flow cytometry analysis. Int. J. Mol. Sci. 21 (9), 3035.
Martyanov A.A., Balabin F.A., Dunster J.L., Panteleev M.A., Gibbins J.M., Sveshnikova A.N. 2020. Control of platelet CLEC-2-mediated activation by receptor clustering and tyrosine kinase signaling. Biophys. J. 118 (11), 2641–2655. https://doi.org/10.1016/j.bpj.2020.04.023
Sveshnikova A.N., Balatskiy A.V., Demianova A.S., Shepelyuk T.O., Shakhidzhanov S.S., Balatskaya M.N., Pichugin A.V., Ataullakhanov F.I., Panteleev M.A. 2016. Systems biology insights into the meaning of the platelet’s dual-receptor thrombin signaling. J. Thromb. Haemost. 14 (10), 2045–2057.
Martyanov A.A., Morozova D.S., Khoreva A.L., Panteleev M.A., Shcherbina A.Yu., Sveshnikova A.N. 2020. Features of intracellular calcium signaling of platelets in Wiskott–Aldrich syndrome. Voprosy Onkol./Gematol. Immunopatol. v Pediatrii (Rus.). 19 (1), 100–107.
Furihata K., Clemetson K.J., Deguchi H., Kunicki T.J. 2001. Variation in human platelet glycoprotein VI content modulates glycoprotein VI-specific prothrombinase activity. Arterioscler. Thromb. Vasc. Biol. 21 (11), 1857–1863.
Best D., Senis Y.A., Jarvis G.E., Eagleton H.J., Roberts D.J., Saito T., Jung S.M., Moroi M., Harrison P., Green F.R., Watson S.P. 2003. GPVI levels in platelets: Relationship to platelet function at high shear. Blood. 102 (8), 2811–2818.
Joutsi-Korhonen L., Smethurst P.A., Rankin A., Gray E., IJsseldijk M., Onley C.M., Watkins N.A., Williamson L.M., Goodall A.H., de Groot P.G., Farndale R.W., Ouwehand W.H. 2003. The low-frequency allele of the platelet collagen signaling receptor glycoprotein VI is associated with reduced functional responses and expression. Blood. 101 (11), 4372–4379.
Croft S.A., Samani N.J., Teare M.D., Hampton K.K., Steeds K.S., Channer K.S., Daly M.E. 2001. Novel platelet membrane glycoprotein VI dimorphism is a risk factor for myocardial infarction. Circulation. 104 (13), 1459–1463.
Trifiro E., Williams S.A., Cheli Y., Furihata K., Pulcinelli F.M., Nugent D.J., Kunicki T.J. 2009. The low-frequency isoform of platelet glycoprotein VIb attenuates ligand-mediated signal transduction but not receptor expression or ligand binding. Blood. 114 (9), 1893–1899.
Jones C.I., Garner S.F., Angenent W., Bernard A., Berzuini C., Burns P., Farndale R.W., Hogwood J., Rankin A., Stephens J.C., Tom B.D., Walton J., Dudbridge F., Ouwehand W.H., Goodall A.H. 2007. Mapping the platelet profile for functional genomic studies and demonstration of the effect size of the GP6 locus. J. Thromb. Haemost. 5 (8), 1756–1765.
Ollikainen E., Mikkelsson J., Perola M., Penttilä A., Karhunen P.J. 2004. Platelet membrane collagen receptor glycoprotein VI polymorphism is associated with coronary thrombosis and fatal myocardial infarction in middle-aged men. Atherosclerosis. 176 (1), 95–99.
Takagi S., Iwai N., Baba S., Mannami T., Ono K., Tanaka C., Miyata T., Miyazaki S., Nonogi H., Goto Y. 2002. A GPVI polymorphism is a risk factor for myocardial infarction in Japanese. Atherosclerosis. 165 (2), 397–398.
Snoep J.D., Gaussem P., Eikenboom J.C.J., Emmerich J., Zwaginga J.J., Holmes C.E., Vos H.L., De Groot P.H.G., Herrington D.M., Bray P.F., Rosendaal F.R., Van der Bom J.G. 2010. The minor allele of GP6 T13254C is associated with decreased platelet activation and a reduced risk of recurrent cardiovascular events and mortality: Results from the SMILE–Platelets project. J. Thromb. Haemost. 8 (11), 2377–2384.
Bernardi B., Guidetti G.F., Campus F., Crittenden J.R., Graybiel A.M., Balduini C., Torti M. 2006. The small GTPase Rap1b regulates the cross talk between platelet integrin alpha2beta1 and integrin alphaIIbbeta3. Blood. 107 (7), 2728–2735.
Bertoni A., Tadokoro S., Eto K., Pampori N., Parise L.V., White G.C., Shattil S.J. 2002. Relationships between Rap1b, affinity modulation of integrin αIIbβ3, and the actin cytoskeleton. J. Biol. Chem. 277 (28), 25 715–25 721.
Gilio K., Munnix I.C.A., Mangin P., Cosemans J.M.E.M., Feijge M.A.H., van der Meijden P.E.J., Olieslagers S., Chrzanowska-Wodnicka M.B., Lillian R., Schoenwaelder S., Koyasu S., Sage S.O., Jackson S.P., Heemskerk J.W.M. 2009. Non-redundant roles of phosphoinositide 3-kinase isoforms alpha and beta in glycoprotein VI-induced platelet signaling and thrombus formation. J. Biol. Chem. 284 (49), 33 750–33 762.
Gardiner E.E., Karunakaran D., Shen Y., Arthur J.F., Andrews R.K., Berndt M.C. 2007. Controlled shedding of platelet glycoprotein (GP)VI and GPIb–IX–V by ADAM family metalloproteinases. J. Thromb. Haemost. 5 (7), 1530–1537.
Andrews R.K., Karunakaran D., Gardiner E.E., Berndt M.C. 2007. Platelet receptor proteolysis: A mechanism for downregulating platelet reactivity. Arterioscler. Thromb. Vasc. Biol. 27 (7), 1511–1520.
Stephens G., Yan Y., Jandrot-Perrus M., Villeval J.L., Clemetson K.J., Phillips D.R. 2005. Platelet activation induces metalloproteinase-dependent GP VI cleavage to down-regulate platelet reactivity to collagen. Blood. 105 (1), 186–191.
Massberg S., Konrad I., Bültmann A., Schulz C., Münch G., Peluso M., Lorenz M., Schneider S., Besta F., Müller I., Hu B.I.N., Langer H., Kremmer E., Rudelius M., Heinzmann U., Ungerer M., Gawaz M. 2003. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 18 (2), 397–399.
Alessi M.C., Sié P., Payrastre B. 2020. Strengths and weaknesses of light transmission aggregometry in diagnosing hereditary platelet function disorders. J. Clin. Med. 9 (3), 763.
ACKNOWLEDGMENTS
The authors are grateful to M.A. Panteleev (CTP PCP RAS, Moscow, Russia) for discussions during the research and V.V. Popov (Faculty of Fundamental Medicine, Moscow State University, Russia) for advice on animal research.
Funding
This work was supported by the Russian Science Foundation (project no. 17-74-20 045).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
All experimental procedures were performed in accordance with the Helsinki Declaration and Directive of the European Parliament 63/2010/EU on humane treatment of animals and were approved by the Ethics Committee of the TTC FHF RAS. In studies involving humans, an informed consent was obtained from all individual participants.
Additional information
Translated by A. Martyanov
Abbreviations: CRP, collagen-related peptide; EPR, endoplasmic reticulum; GPVI, glycoprotein-VI; PRP, platelet rich plasma; vWF, Willebrand factor.
Rights and permissions
About this article
Cite this article
Stepanyan, M.G., Filkova, A.A., Garzon Dasgupta, A.K. et al. Platelet Activation through GPVI Receptor: Variability of the Response. Biochem. Moscow Suppl. Ser. A 15, 73–81 (2021). https://doi.org/10.1134/S1990747820050074
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747820050074