Abstract
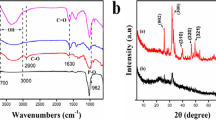
Millimetric spherical beads of a biocompatible composite were produced from sodium alginate, a natural polysaccharide, and nanostructured hydroxyapatite (HA). It was shown that the composite was effective in the removal of lead ions and lead phosphate nanoparticles from high-contaminated simulated gastric fluid. X-ray diffraction spectroscopy and scanning electron microscopy analyses performed on HA–alginate beads after the Pb2+ uptake showed that nanocrystals of a lead phosphate, [Pb10–xCax(PO4)6Cl2], were precipitated on the bead surface. The cross-linked polymer chain had a double role: (i) keep Pb2+ ions and lead phosphate nanoparticles bounded to the bead surface, preventing their bioavailability in stomach fluid; and (ii) delay HA dissolution in the acidic conditions of the stomach, assuring that an excess of Ca2+ will not be released to simulated gastric fluid. Desorption experiments in simulated enteric fluid revealed that lead remained immobilized in the calcium phosphate phase in the intestinal tract. These results indicate HA–alginate composite as a potential system for heavy metals removal from contaminated gastric and enteric human fluids, minimizing its adsorption by the human body.







Similar content being viewed by others
References
Environmental Protection Agency: http://www.environment.nsw.gov.au/leadsafe/leadinf3.htm.
E. Deydier, R. Guilet, and P. Sharrock: Beneficial use of meat and bone meal combustion residue: An efficient low cost material to remove lead from aqueous effluent. J. Hazard. Mater. B 101, 55 (2003).
G. Levy and K. Rao: Enhanced intestinal absorption of riboflavin from sodium alginate solution in man. J. Pharm. Sci. 61, 279 (1972).
D.J. Park, B.H. Choi, S.J. Zhuh, J.Y. Huh, B.Y. Kim, and S.H. Lee: Injectable bone using chitosan-alginate gel/mesenchymal stem cells/BMP-2 composites. J. Craniomaxillofac. Surg. 33, 50 (2005).
C.C. Barrias, C.C. Ribeiro, M. Lamghari, C.S. Miranda, and M.A. Barbosa: Proliferation, activity, and osteogenic differentiation of bone marrow stromal cells cultured on calcium titanium phosphate microspheres. J. Biomed. Mater. Res. A 72(1), 57 (2005).
E. Krylova, A. Ivanov, V. Orlovsk, G. El-Registan, and S. Barinov: Hydroxyapatite-polysaccharide granules for drug delivery. J. Mater. Sci.: Mater. Med. 13, 87 (2002).
B. Buranapanitkit, K. Oungbho, and N. Ingviya: The efficacy of hydroxyapatite composite impregnated with amphotericin B. Clin. Orthop. Relat. Res. 437, 236 (2005).
T. Gotoh, K. Matsushima, and K.I. Kikuchi: Adsorption of Cu and Mn on covalently cross-liked alginate gel beads. Chemosphere 55, 57 (2004).
E. Mavropoulos, A.M. Rossi, A.M. Costa, C.A.C. Perez, and J.C. Moreira: Studies on the mechanisms of lead immobilization by hydroxyapatite. Environ. Sci. Technol. 36, 1625 (2002).
N.C.C. Rocha, R.C. Campos, A.M. Rossi, E.L. Moreira, A.F. Barbosa, and G.T. Moure: Cadmium uptake by hydroxyapatite synthesized in different conditions and submitted to thermal treatment. Environ. Sci. Technol. 36, 1630 (2002).
N. Arnich, M.C. Lanhers, F. Laurensot, R. Podor, A. Montiel, and D. Burnel: In vitro and in vivo studies of lead immobilization by synthetic hydroxyapatite. Environ. Pollut. 124, 139 (2003).
E. Hayek and H. Newesely: Pentacalcium monohydroxyorthophosphate. Inorg. Synth. 7, 63 (1963).
P.V. Finotelli, M.A. Morales, M.H. Rocha-Leão, E.M. Baggio-Saitovitch, and A.M. Rossi: Magnetic studies of iron (III) nanoparticles in alginate polymer for drug delivery applications. Mater. Sci. Eng., C 24, 625 (2004).
J.O. Nriagu: Lead orthophosphates II: Stability of cloropyromorphite at 25 °C. Geochim. Cosmochim. Acta 37, 367 (1973).
Q.Y. Ma, S.J. Traina, T.J. Logan, and J.A. Ryan: In situ lead immobilization by apatite. Environ. Sci. Technol. 27, 1803 (1993).
E. Mavropoulos, N.C.C. Rocha, J.C. Moreira, A.M. Rossi, and G.A. Soares: Characterization of phase evolution during lead immobilization by synthetic hydroxyapatite. Mater. Charact. 53, 71 (2004).
F. Llanes, D.H. Ryan, and R.H. Marchessault: Magnetic nanostructured composites using alginates of different M:G ratios as polymeric matrix. Int.J. Biol. Macromol. 27, 35 (2000).
Acknowledgments
We thank Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Instituto Millitar de Engenharia (IME), Centro de Tecnologia Mineral (CETEM), and Instituto de Ciencias Biomedicas/ Universidade Federal do Rio de Janeiro (ICB/UFRJ) (Dr. Leonardo Andrade) for the SEM analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mavropoulos, E., Rocha-Leão, M.H., da Rocha, N.C.C. et al. Hydroxyapatite-alginate composite for lead removal in artificial gastric fluid. Journal of Materials Research 22, 3371–3377 (2007). https://doi.org/10.1557/JMR.2007.0419
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2007.0419