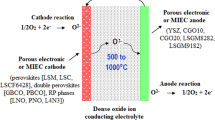
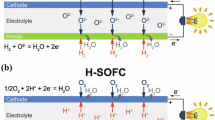
Abstract
Low temperature solid oxide fuel cells (SOFCs) are a promising solution to revolutionize stationary, transportation, and personal power energy conversion efficiency. Through investigation of fundamental conduction mechanisms, we have developed the highest conductivity solid electrolyte, stabilized bismuth oxide (Dy0.08W0.04Bi0.88O0.36). To overcome its inherent thermodynamic instability in the anode environment, we invented a functionally graded bismuth oxide/ceria bilayered electrolyte. For compatibility with this bilayared electrolyte, we developed high performance bismuth ruthenate–bismuth oxide composite cathodes. Finally, these components were integrated into an anode-supported cell with an anode functional layer, resulting in an exceptionally high power density of ∼2 W/cm2 at moderate temperatures (650 °C) and sufficient power down to 300–400 °C for most applications. Moreover, because SOFCs can operate on conventional fuels, these low temperature SOFCs provide one of the most efficient energy conversion technologies available without relying on a hydrogen infrastructure.

















Similar content being viewed by others
References
E.D. Wachsman and K.T. Lee: Lowering the temperature of solid oxide fuel cells. Science 334, 935 (2011).
E.D. Wachsman, C.A. Marlowe, and K.T. Lee: Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 5, 5498 (2012).
B.C.H Steele: Material science and engineering: The enabling technology for the commercialisation of fuel cell systems. J. Mater. Sci. 36, 1053 (2001).
E.D. Wachsman, N. Jiang, D.M. Mason, and D.A. Stevenson: Solid state oxygen kinetics in Er2O3 stabilized Bi2O3. Proc. Electrochem. Soc. 89, 15 (1989).
E.D. Wachsman, N. Jiang, C.W. Frank, D.M. Mason, and D.A. Stevenson: Spectroscopic investigation of oxygen vacancies in solid oxide electrolytes. Appl. Phys. A: Mater. Sci. Process. 50, 545 (1990).
E.D. Wachsman, G.R. Ball, N. Jiang, and D.A. Stevenson: Structural and defect studies in solid oxide electrolytes. Solid State Ionics 52, 213 (1992).
N. Jiang, R.M. Buchanan, F.E.G Henn, A.F. Marshall, D.A. Stevenson, and E.D. Wachsman: Aging phenomenon of stabilized bismuth oxides. Mater. Res. Bull. 29, 247 (1994).
N.X. Jiang and E.D. Wachsman: Structural stability and conductivity of phase-stabilized cubic bismuth oxides. J. Am. Ceram. Soc. 82, 3057 (1999).
E.D. Wachsman, S. Boyapati, M.J. Kaufman, and N.X. Jiang: Modeling of ordered structures of phase-stabilized cubic bismuth oxides. J. Am. Ceram. Soc. 83, 1964 (2000).
S. Boyapati, E.D. Wachsman, and N.X. Jiang: Effect of oxygen sublattice ordering on interstitial transport mechanism and conductivity activation energies in phase-stabilized cubic bismuth oxides. Solid State Ionics 140, 149 (2001).
S. Boyapati, E.D. Wachsman, and B.C. Chakoumakos: Neutron diffraction study of occupancy and positional order of oxygen ions in phase stabilized cubic bismuth oxides. Solid State Ionics 138, 293 (2001).
E.D. Wachsman, S. Boyapati, and N. Jiang: Effect of dopant polarizability on oxygen sublattice order in phase-stable cubic bismuth oxide. Ionics 7, 6 (2001).
N.X. Jiang, E.D. Wachsman, and S.H. Jung: A higher conductivity Bi2O3-based electrolyte. Solid State Ionics 150, 347 (2002).
E.D. Wachsman: Effect of oxygen sublattice order on conductivity in highly defective fluorite oxides. J. Eur. Ceram. Soc. 24, 1281 (2004).
D.W. Jung, K.L. Duncan, and E.D. Wachsman: Effect of total dopant concentration and dopant ratio on conductivity of (DyO1.5)x-(WO3)y-(BiO1.5)1-x-y. Acta Mater. 58, 355 (2010).
D.W. Jung, J.C. Nino, K.L. Duncan, S.R. Bishop, and E.D. Wachsman: Enhanced long-term stability of bismuth oxide-based electrolytes for operation at 500 A degrees C. Ionics 16, 97 (2010).
D.W. Jung, K.L. Duncan, M.A. Camaratta, K.T. Lee, J.C. Nino, and E.D. Wachsman: Effect of annealing temperature and dopant concentration on the conductivity behavior in (DyO1.5)x-(WO3)y-(BiO1.5)1-x-y. J. Am. Ceram. Soc. 93, 1384 (2010).
E.D. Wachsman, P. Jayaweera, N. Jiang, D.M. Lowe, and B.G. Pound: Stable high conductivity ceria/bismuth oxide bilayered electrolytes. J. Electrochem. Soc. 144, 233 (1997).
A. Jaiswal, C.T. Hu, and E.D. Wachsman: Bismuth ruthenate-stabilized bismuth oxide composite cathodes for IT-SOFC. J. Electrochem. Soc. 154, B1088 (2007).
M. Camaratta and E. Wachsman: High-performance composite Bi2Ru2O7-Bi1.6Er0.4O3 cathodes for intermediate-temperature solid oxide fuel cells. J. Electrochem. Soc. 155, B135 (2008).
J.S. Ahn, M.A. Camaratta, D. Pergolesi, K.T. Lee, H. Yoon, B.W. Lee, D.W. Jung, E. Traversa, and E.D. Wachsman: Development of high performance ceria/bismuth oxide bilayered electrolyte SOFCs for lower temperature operation. J. Electrochem. Soc. 157, B376 (2010).
J.S. Ahn, D. Pergolesi, M.A. Camaratta, H. Yoon, B.W. Lee, K.T. Lee, D.W. Jung, E. Traversa, and E.D. Wachsman: High-performance bilayered electrolyte intermediate temperature solid oxide fuel cells. Electrochem. Commun. 11, 1504 (2009).
B.C.H Steele and A. Heinzel: Materials for fuel-cell technologies. Nature 414, 345 (2001).
S. deSouza, S.J. Visco, and L.C. DeJonghe: Thin-film solid oxide fuel cell with high performance at low-temperature. Solid State Ionics 98, 57 (1997).
B.C.H Steele: Interfacial reactions associated with ceramic ion-transport membranes. Solid State Ionics 75, 157 (1995).
T. Takahashi, T. Esaka, and H. Iwahara: High oxide ion conduction in sintered oxides of system Bi2O3-Gd2O3. J. Appl. Electrochem. 5, 197 (1975).
H.A. Harwig: Structure of bismuthsesquioxide–alpha, beta, gamma and delta-phase. Z. Anorg. Allg. Chem. 444, 151 (1978).
T. Takahashi, T. Esaka, and H. Iwahara: Electrical-conduction in sintered oxides of system Bi2O3-BaO. J. Solid State Chem. 16, 317 (1976).
M.J. Verkerk, K. Keizer, and A.J. Burggraaf: High oxygen ion conduction in sintered oxides of the Bi2O3-Er2O3 system. J. Appl. Electrochem. 10, 81 (1980).
L.G. Sillen: X-ray studies on bismuth trioxide. Ark. Kemi Mineral. Geol. 12A, 15 (1937).
G. Gattow and H. Schröder: About bismuth oxides. III. The crystal structure of the high-temperature modification of bismuth (III) oxide (δ-Bi2O3). Z. Anorg. Allg. Chem. 318, 14 (1962).
B.T.M Willis: The anomalous behaviour of the neutron reflexion of fluorite. Acta Crystallogr. 18, 2 (1965).
D.S. Aidhy, J.C. Nino, S.B. Sinnott, E.D. Wachsman, and S.R. Phillpot: Vacancy-ordered structure of cubic bismuth oxide from simulation and crystallographic analysis. J. Am. Ceram. Soc. 91, 2349 (2008).
D.S. Aidhy, S.B. Sinnott, E.D. Wachsman, S.R. Phillpot, and J.C. Nino: Structure of delta-Bi2O3 from density functional theory: A systematic crystallographic analysis. J. Solid State Chem. 182, 1222 (2009).
S.N. Hoda and L.L.Y Chang: Phase relations in system Bi2O3-WO3. J. Am. Ceram. Soc. 57, 323 (1974).
A. Watanabe and A. Ono: Thermostable region of an oxide ion conductor, Bi7WO13.5 (=7Bi2O3 -2WO3), and the solid solubility extension. Solid State Ionics 174, 15 (2004).
T. Takahashi, T. Esaka, and H. Iwahara: Conduction in Bi2O3-based oxide ion conductors under low oxygen-pressure. 1. Current blackening of Bi2O3-Y2O3 electrolyte. J. Appl. Electrochem. 7, 299 (1977).
C.Z. Wang, X.G. Xu, and B.Z. Li: Ionic and electronic conduction of oxygen ion conductors in the Bi2O3-Y2O3 system. Solid State Ionics 13, 135 (1984).
P. Duran, J.R. Jurado, C. Moure, N. Valverde, and B.C.H Steele: High oxygen ion conduction in some Bi2O3-Y2O3(Er2O3) solid-solutions. Mater. Chem. Phys. 18, 287 (1987).
H. Yahiro, Y. Eguchi, K. Eguchi, and H. Arai: Oxygen ion conductivity of the ceria samarium oxide system with fluorite structure. J. Appl. Electrochem. 18, 527 (1988).
K. Eguchi, T. Setoguchi, T. Inoue, and H. Arai: Electrical-properties of ceria-based oxides and their application to solid oxide fuel-cells. Solid State Ionics 52, 165 (1992).
B.C.H Steele: Appraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500 degrees C. Solid State Ionics 129, 95 (2000).
S. Omar, E.D. Wachsman, and J.C. Nino: A co-doping approach towards enhanced ionic conductivity in fluorite-based electrolytes. Solid State Ionics 177, 3199 (2006).
S. Omar, E.D. Wachsman, and J.C. Nino: Higher ionic conductive ceria-based electrolytes for solid oxide fuel cells. Appl. Phys. Lett. 91, 114106–1–114106–3 (2007).
S. Omar, E.D. Wachsman, and J.C. Nino: Higher conductivity Sm3+ and Nd3+ co-doped ceria-based electrolyte materials. Solid State Ionics 178, 1890 (2008).
D.A. Andersson, S.I. Simak, N.V. Skorodumova, I.A. Abrikosov, and B. Johansson: Optimization of ionic conductivity in doped ceria. Proc. Natl. Acad. Sci. U.S.A. 103, 3518 (2006).
R.D. Shannon: Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., Sect. A 32, 751 (1976).
J.S. Ahn, S. Omar, H. Yoon, J.C. Nino, and E.D. Wachsman: Performance of anode-supported solid oxide fuel cell using novel ceria electrolyte. J. Power Sources 195, 2131 (2010).
K.L. Duncan and E.D. Wachsman: Continuum-level analytical model for solid oxide fuel cells with mixed conducting electrolytes. J. Electrochem. Soc. 156, B1030 (2009).
S.M. Haile: Fuel cell materials and components. Acta Mater. 51, 5981 (2003).
K.T. Lee, N.J. Vito, C.A. Mattehw, H.S. Yoon, and E.D. Wachsman: Effect of Ni-GDC AFL composition on performance of IT-SOFCs. ECS Trans. 28, 151 (2010).
D.J.L Brett, A. Atkinson, N.P. Brandon, and S.J. Skinner: Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 37, 1568 (2008).
X. Zhang, M. Robertson, C. Deces-Petit, W. Qu, O. Kesler, R. Maric, and D. Ghosh: Internal shorting and fuel loss of a low temperature solid oxide fuel cell with SDC electrolyte. J. Power Sources 164, 668 (2007).
K.L. Duncan, K.T. Lee, and E.D. Wachsman: Dependence of open-circuit potential and power density on electrolyte thickness in solid oxide fuel cells with mixed conducting electrolytes. J. Power Sources 196, 2445 (2011).
J.Y. Park and E.D. Wachsman: Stable and high conductivity ceria/bismuth oxide bilayer electrolytes for lower temperature solid oxide fuel cells. Ionics 12, 15 (2006).
E.D. Wachsman: Functionally gradient bilayer oxide membranes and electrolytes. Solid State Ionics 152, 657 (2002).
K.T. Lee, D.W. Jung, M.A. Camaratta, J.S. Ahn, and E.D. Wachsman: Gd0.1Ce0.9O1.95/Er0.2Bi1.6O3 bilayered electrolytes fabricated by a simple colloidal route using nano-sized Er0.2Bi1.6O3 powders for high performance LT-SOFCs. J. Power Sources 205, 122 (2012).
Q.L. Liu, K.A. Khor, S.H. Chan, and X.J. Chen: Anode-supported solid oxide fuel cell with yttria-stabilized zirconia/gadolinia-doped ceria bilayer electrolyte prepared by wet ceramic co-sintering process. J. Power Sources 162, 1036 (2006).
H.T. Lim and A.V. Virkar: Measurement of oxygen chemical potential in Gd2O3-doped ceria-Y2O3-stabilized zirconia bi-layer electrolyte, anode-supported solid oxide fuel cells. J. Power Sources 192, 267 (2009).
C.R. Xia, W. Rauch, F.L. Chen, and M.L. Liu: Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs. Solid State Ionics 149, 11 (2002).
K. Sasaki, J. Tamura, H. Hosoda, T.N. Lan, K. Yasumoto, and M. Dokiya: Pt-perovskite cermet cathode for reduced-temperature SOM. Solid State Ionics 148, 551 (2002).
T. Ishihara, T. Kudo, H. Matsuda, and Y. Takita: Doped PrMnO3 perovskite oxide as a new cathode of solid oxide fuel-cells for low-temperature operation. J. Electrochem. Soc. 142, 1519 (1995).
M. Mogensen and S. Skaarup: Kinetic and geometric aspects of solid oxide fuel cell electrodes. Solid State Ionics 86–, 1151 (1996).
M. Godickemeier, K. Sasaki, L.J. Gauckler, and I. Riess: Perovskite cathodes for solid oxide fuel cells based on ceria electrolytes. Solid State Ionics 86–, 691 (1996).
N.Q. Minh: Ceramic fuel-cells. J. Am. Ceram. Soc. 76, 563 (1993).
E.P. Murray, T. Tsai, and S.A. Barnett: Oxygen transfer processes in (La, Sr)MnO3/Y2O3-stabilized ZrO2 cathodes: An impedance spectroscopy study. Solid State Ionics 110, 235 (1998).
S.P. Yoon, J. Han, S.W. Nam, T.H. Lim, I.H. Oh, S.A. Hong, Y.S. Yoo, and H.C. Lim: Performance of anode-supported solid oxide fuel cell with La0.85Sr0.15MnO3 cathode modified by sol-gel coating technique. J. Power Sources 106, 160 (2002).
S.P. Jiang: Issues on development of (La, Sr)MnO3 cathode for solid oxide fuel cells. J. Power Sources 124, 390 (2003).
N.P. Brandon, S. Skinner, and B.C.H Steele: Recent advances in materials for fuel cells. Annu. Rev. Mater. Res. 33, 183 (2003).
C.W. Tanner, K.Z. Fung, and A.V. Virkar: The effect of porous composite electrode structure on solid oxide fuel cell performance. 1. Theoretical analysis. J. Electrochem. Soc. 144, 21 (1997).
E.P. Murray and S.A. Barnett: (La, Sr) MnO3-(Ce, Gd)O2-x composite cathodes for solid oxide fuel cells. Solid State Ionics 143, 265 (2001).
S.P. Jiang: A comparison of O−2 reduction reactions on porous (La, Sr)MnO3 and (La, Sr)(Co, Fe)O3 electrodes. Solid State Ionics 146, 1 (2002).
I. Yasuda, K. Ogasawara, M. Hishinuma, T. Kawada, and M. Dokiya: Oxygen tracer diffusion coefficient of (La, Sr)MnO3+/-delta. Solid State Ionics 86–, 1197 (1996).
C.C. Kan, H.H. Kan, F.M. Van Assche, E.N. Armstrong, and E.D. Wachsman: Investigating oxygen surface exchange kinetics of La0.8Sr0.2MnO3-delta and La0.6Sr0.4Co0.2Fe0.8O3-delta using an isotopic tracer. J. Electrochem. Soc. 155, B985 (2008).
S.B. Adler: Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 104, 4791 (2004).
J.M. Vohs and R.J. Gorte: High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 21, 943 (2009).
L. Baque, A. Caneiro, M.S. Moreno, and A. Serquis: High performance nanostructured IT-SOFC cathodes prepared by novel chemical method. Electrochem. Commun. 10, 1905 (2008).
J.R. Wilson and S.A. Barnett: Solid oxide fuel cell Ni-YSZ anodes: Effect of composition on microstructure and performance. Electrochem. Solid-State Lett. 11, B181 (2008).
N. Shikazono, Y. Sakamoto, Y. Yamaguchi, and N. Kasagi: Microstructure and polarization characteristics of anode supported tubular solid oxide fuel cell with co-precipitated and mechanically mixed Ni-YSZ anodes. J. Power Sources 193, 530 (2009).
A. Bieberle, L.P. Meier, and L.J. Gauckler: The electrochemistry of Ni pattern anodes used as solid oxide fuel cell model electrodes. J. Electrochem. Soc. 148, A646 (2001).
J.R. Wilson, W. Kobsiriphat, R. Mendoza, H.Y. Chen, J.M. Hiller, D.J. Miller, K. Thornton, P.W. Voorhees, S.B. Adler, and S.A. Barnett: Three-dimensional reconstruction of a solid-oxide fuel-cell anode. Nat. Mater. 5, 541 (2006).
D. Gostovic, J.R. Smith, D.P. Kundinger, K.S. Jones, and E.D. Wachsman: Three-dimensional reconstruction of porous LSCF cathodes. Electrochem. Solid-State Lett. 10, B214 (2007).
J.R. Smith, A. Chen, D. Gostovic, D. Hickey, D. Kundinger, K.L. Duncan, R.T. DeHoff, K.S. Jones, and E.D. Wachsman: Evaluation of the relationship between cathode microstructure and electrochemical behavior for SOFCs. Solid State Ionics 180, 90 (2009).
J.R. Wilson, A.T. Duong, M. Gameiro, H.Y. Chen, K. Thornton, D.R. Mumm, and S.A. Barnett: Quantitative three-dimensional microstructure of a solid oxide fuel cell cathode. Electrochem. Commun. 11, 1052 (2009).
J.R. Wilson, M. Gameiro, K. Mischaikow, W. Kalies, P.W. Voorhees, and S.A. Barnett: Three-dimensional analysis of solid oxide fuel cell Ni-YSZ anode interconnectivity. Microsc. Microanal. 15, 71 (2009).
J.R. Wilson, J.S. Cronin, A.T. Duong, S. Rukes, H.Y. Chen, K. Thornton, D.R. Mumm, and S. Barnett: Effect of composition of (La0.8Sr0.2MnO3-Y2O3-stabilized ZrO2) cathodes: Correlating three-dimensional microstructure and polarization resistance. J. Power Sources 195, 1829 (2010).
N. Shikazono, D. Kanno, K. Matsuzaki, H. Teshima, S. Sumino, and N. Kasagi: Numerical assessment of SOFC anode polarization based on three-dimensional model microstructure reconstructed from FIB-SEM images. J. Electrochem. Soc. 157, B665 (2010).
C.C. Kan and E.D. Wachsman: Identifying drivers of catalytic activity through systematic surface modification of cathode materials. J. Electrochem. Soc. 156, B695 (2009).
C.C. Kan and E.D. Wachsman: Isotopic-switching analysis of oxygen reduction in solid oxide fuel cell cathode materials. Solid State Ionics 181, 338 (2010).
Z.P. Shao and S.M. Haile: A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170 (2004).
A. Jaiswal and E.D. Wachsman: Bismuth-ruthenate-based cathodes for IT-SOFCs. J. Electrochem. Soc. 152, A787 (2005).
A. Jaiswal and E. Wachsman: Impedance studies on bismuth-ruthenate-based electrodes. Ionics 15, 1 (2009).
K.S. Lee, D.K. Seo, and M.H. Whangbo: Structural and electronic factors governing the metallic and nonmetallic properties of the pyrochlores A2Ru2O7-y. J. Solid State Chem. 131, 405 (1997).
M. Camaratta and E. Wachsman: Silver-bismuth oxide cathodes for IT-SOFCs - Part II - Improving stability through microstructural control. Solid State Ionics 178, 1411 (2007).
M. Camaratta and E. Wachsman: Silver-bismuth oxide cathodes for IT-SOFCs; Part I - Microstructural instability. Solid State Ionics 178, 1242 (2007).
J. Will, A. Mitterdorfer, C. Kleinlogel, D. Perednis, and L.J. Gauckler: Fabrication of thin electrolytes for second-generation solid oxide fuel cells. Solid State Ionics 131, 79 (2000).
J.W. Kim, A.V. Virkar, K.Z. Fung, K. Mehta, and S.C. Singhal: Polarization effects in intermediate temperature, anode-supported solid oxide fuel cells. J. Electrochem. Soc. 146, 69 (1999).
J.S. Ahn, H. Yoon, K.T. Lee, M.A. Camaratta, and E.D. Wachsman: Performance of IT-SOFC with Ce0.9Gd0.1O1.95 functional layer at the interface of Ce0.9Gd0.1O1.95 electrolyte and Ni-Ce0.9Gd0.1O1.95 anode. Fuel Cells 9, 643 (2009).
N. Ai, Z. Lu, K.F. Chen, X.Q. Huang, X.B. Du, and W.H. Su: Effects of anode surface modification on the performance of low temperature SOFCs. J. Power Sources 171, 489 (2007).
J.J. Haslam, A.Q. Pham, B.W. Chung, J.F. DiCarlo, and R.S. Glass: Effects of the use of pore formers on performance of an anode supported solid oxide fuel cell. J. Am. Ceram. Soc. 88, 513 (2005).
N. Ai, Z. Lu, J.K. Tang, K.F. Chen, X.Q. Huang, and W.H. Su: Improvement of output performance of solid oxide fuel cell by optimizing Ni/samaria-doped ceria anode functional layer. J. Power Sources 185, 153 (2008).
D. Stover, H.P. Buchkremer, and S. Uhlenbruck: Processing and properties of the ceramic conductive multilayer device solid oxide fuel cell (SOFC). Ceram. Int. 30, 1107 (2004).
S.D. Kim, S.H. Hyun, J. Moon, J.H. Kim, and R.H. Song: Fabrication and characterization of anode-supported electrolyte thin films for intermediate temperature solid oxide fuel cells. J. Power Sources 139, 67 (2005).
E. Wanzenberg, F. Tietz, P. Panjan, and D. Stover: Influence of pre- and post-heat treatment of anode substrates on the properties of DC-sputtered YSZ electrolyte films. Solid State Ionics 159, 1 (2003).
K.T. Lee, H.S. Yoon, J.S. Ahn, and E.D. Wachsman: Bimodally integrated Ni-Gd0.1Ce0.9O1.95 anode functional layer for lower temperature SOFCs. Int. J. Hydrogen Energy (2012, submitted).
C.W. Sun and U. Stimming: Recent anode advances in solid oxide fuel cells. J. Power Sources 171, 247 (2007).
E.P. Murray, T. Tsai, and S.A. Barnett: A direct-methane fuel cell with a ceria-based anode. Nature 400, 649 (1999).
Z.L. Zhan and S.A. Barnett: An octane-fueled solid oxide fuel cell. Science 308, 844 (2005).
L. Yang, S.Z. Wang, K. Blinn, M.F. Liu, Z. Liu, Z. Cheng, and M.L. Liu: Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2-xYbxO3-delta. Science 326, 126 (2009).
S.D. Park, J.M. Vohs, and R.J. Gorte: Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 404, 265 (2000).
R.J. Gorte and J.M. Vohs: Nanostructured anodes for solid oxide fuel cells. Curr. Opin. Colloid Interface Sci. 14, 236 (2009).
J. Pena-Martinez, D. Marrero-Lopez, J.C. Ruiz-Morales, C. Savaniu, P. Nunez, and J.T.S Irvine: Anodic performance and intermediate temperature fuel cell testing of La0.75Sr0.25Cr0.5Mn0.5O3-delta at lanthanum gallate electrolytes. Chem. Mater. 18, 1001 (2006).
Q.X. Fu, F. Tietz, and D. Stover: La0.4Sr0.6Ti1-xMnxO3-delta perovskites as anode materials for solid oxide fuel cells. J. Electrochem. Soc. 153, D74 (2006).
Y.H. Huang, R.I. Dass, Z.L. Xing, and J.B. Goodenough: Double perovskites as anode materials for solid-oxide fuel cells. Science 312, 254 (2006).
K.T. Lee, C.M. Gore, and E.D. Wachsman: Performance of lower temperature solid oxide fuel cells operating on reformed hydrocarbon fuels. J. Power Sources (2012, submitted).
K.T. Lee, D.W. Jung, H.S. Yoon, M. Camaratta, N. Sexson, and E. Wachsman: High performance LSM-ESB cathode on ESB electrolyte for low to intermediate temperature solid oxide fuel cells. ECS Trans. 35, 1861 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper has been selected as an Invited Feature Paper.
Rights and permissions
About this article
Cite this article
Lee, K.T., Yoon, H.S. & Wachsman, E.D. The evolution of low temperature solid oxide fuel cells. Journal of Materials Research 27, 2063–2078 (2012). https://doi.org/10.1557/jmr.2012.194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.194