Abstract
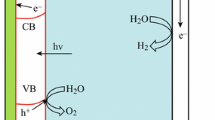
Photoanodes based on TiO2-polyheptazine (TiO2-PH) hybrids are, due to the energetics of photogenerated charges, very promising for solar water splitting in terms of possibly reduced need for external electric bias. Visible (λ > 420 nm) light-driven photooxidation of water at TiO2-PH electrodes loaded with two different metal oxide cocatalysts was investigated. As compared with TiO2-PH photoanodes loaded with colloidal [iridium (IV) oxide] IrO2 deposited by colloidal deposition, photoelectrodes modified with CoOx oxygen-evolving cocatalyst (Co-Pi) deposited by photoassisted deposition precipitation method showed both higher photocurrents and more efficient oxygen evolution under prolonged irradiation. The minimum external electric bias needed to observe complete photooxidation of water to dioxygen at TiO2-PH photoanodes modified with Co-Pi was estimated to be ∼0.6 V at pH 7. The key factor limiting the photoconversion efficiency at low bias potentials is the fast primary recombination of photogenerated charges.






Similar content being viewed by others
References
N.S. Lewis and D.G. Nocera: Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. U.S.A. 103, 15729 (2006).
O. Khaselev and J.A. Turner: A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280, 425 (1998).
A. Heller: Conversion of sunlight into electrical power and photoassisted electrolysis of water in photoelectrochemical cells. Acc. Chem. Res. 14, 154 (1981).
H.J. Lewerenz, C. Heine, K. Skorupska, N. Szabo, T. Hannappel, T. Vo-Dinh, S.A. Campbell, H.W. Klemm, and A.G. Munoz: Photoelectrocatalysis: Principles, nanoemitter applications and routes to bioinspired systems. Energy Environ. Sci. 3, 748 (2010).
S. Licht, B. Wang, S. Mukerji, T. Soga, M. Umeno, and H. Tributsch: Over 18% solar energy conversion to generation of hydrogen fuel; theory and experiment for efficient solar water splitting. Int. J. Hydrogen Energy 26, 653 (2001).
H. Dau, C. Limberg, T. Reier, M. Risch, S. Roggan, and P. Strasser: The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. ChemCatChem 2, 724 (2010).
J. Rossmeisl, Z.W. Qu, H. Zhu, G.J. Kroes, and J.K. Norskov: Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83 (2007).
A. Valdés, Z.W. Qu, G.J. Kroes, J. Rossmeisl, and J.K. Nørskov: Oxidation and photooxidation of water on TiO2 surface. J. Phys. Chem. C 112, 9872 (2008).
H. Wang, T. Deutsch, and J.A. Turner: Direct water splitting under visible light with nanostructured hematite and WO3 photoanodes and a GaInP2 photocathode. J. Electrochem. Soc. 155, F91 (2008).
R. van de Krol, Y. Liang, and J. Schoonman: Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 18, 2311 (2008).
B.D. Alexander, P.J. Kulesza, I. Rutkowska, R. Solarska, and J. Augustynski: Metal oxide photoanodes for solar hydrogen production. J. Mater. Chem. 18, 2298 (2008).
W.J. Youngblood, S.-H.A. Lee, Y. Kobayashi, E.A. Hernandez-Pagan, P.G. Hoertz, T.A. Moore, A.L. Moore, D. Gust, and T.E. Mallouk: Photoassisted overall water splitting in a visible light-absorbing dye-sensitized photoelectrochemical Cell. J. Am. Chem. Soc. 131, 926 (2009).
H. Tributsch: Nanocomposite solar cells: The requirement and challenge of kinetic charge separation. J. Solid State Electrochem. 13, 1127 (2009).
H. Dau and I. Zaharieva: Principles, efficiency, and blueprint character of solar-energy conversion in photosynthetic water oxidation. Acc. Chem. Res. 42, 1861 (2009).
J. Rossmeisl, K. Dimitrievski, P. Siegbahn, and J.K. Norskov: Comparing electrochemical and biological water splitting. J. Phys. Chem. C 111, 18821 (2007).
W.J. Youngblood, S.-H.A. Lee, K. Maeda, and T.E. Mallouk: Visible light water splitting using dye-sensitized oxide semiconductors. Acc. Chem. Res. 42, 1966 (2009).
M. Bledowski, L. Wang, A. Ramakrishnan, O.V. Khavryuchenko, V.D. Khavryuchenko, P.C. Ricci, J. Strunk, T. Cremer, C. Kolbeck, and R. Beranek: Visible-light photocurrent response of TiO2-polyheptazine hybrids: Evidence for interfacial charge-transfer absorption. Phys. Chem. Chem. Phys. 13, 21511 (2011).
L. Wang, M. Bledowski, A. Ramakrishnan, D. König, A. Ludwig, and R. Beranek: Dynamics of photogenerated holes in TiO2-polyheptazine hybrid photoanodes for visible light-driven water splitting. J. Electrochem. Soc. 159, H616 (2012).
M. Bledowski, L. Wang, A. Ramakrishnan, A. Betard, O.V. Khavryuchenko, and R. Beranek: Visible-light photooxidation of water to oxygen at hybrid TiO2–polyheptazine photoanodes with photodeposited Co-Pi (CoOx) cocatalyst. ChemPhysChem 13, 3018 (2012).
X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J.M. Carlsson, K. Domen, and M. Antonietti: A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76 (2009).
Y. Wang, X. Wang, and M. Antonietti: Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 51, 68 (2012).
M.W. Kanan and D.G. Nocera: In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072 (2008).
D.K. Zhong, M. Cornuz, K. Sivula, M. Gratzel, and D.R. Gamelin: Photoassisted electrodeposition of cobalt phosphate (Co-Pi) catalyst on hematite photoanodes for solar water oxidation. Energy Environ. Sci. 4, 1759 (2011).
K. Maeda, M. Higashi, B. Siritanaratkul, R. Abe, and K. Domen: SrNbO2N as a water-splitting photoanode with a wide visible-light absorption band. J. Am. Chem. Soc. 133, 12334 (2011).
G. Hodes, I.D.J. Howell, and L.M. Peter: Nanocrystalline photoelectrochemical cells. A new concept in photovoltaic cells. J. Electrochem. Soc. 139, 3136 (1992).
A. Wahl, M. Ulmann, A. Carroy, and J. Augustynski: Highly selective photooxidation reactions at nanocrystalline TiO2 film electrodes. J. Chem. Soc., Chem. Commun. 2277 (1994).
L.M. Peter: Dynamic aspects of semiconductor photoelectrochemistry. Chem. Rev. 90, 753 (1990).
S.D. Tilley, M. Cornuz, K. Sivula, and M. Grätzel: Light-induced water splitting with hematite: Improved nanostructure and iridium oxide catalysis. Angew. Chem. Int. Ed. 49, 6405 (2010).
A. Kay, I. Cesar, and M. Graetzel: New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 128, 15714 (2006).
M. Gratzel: Photoelectrochemical cells. Nature 414, 338 (2001).
R. Beranek: (Photo)electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. (2011) doi:10.1155/2011/786759.
Acknowledgments
We are thankful for financial support by the MIWFT-NRW within the project “Anorganische Nanomaterialien für Anwendungen in der Photokatalyse: Wasseraufbereitung und Wasserstoffgewinnung.” Dr. Pio John Buenconsejo and Prof. Alfred Ludwig are acknowledged for TEM measurements, and Sachtleben Chemie for a free sample of Hombikat UV 100. The support of the Center for Electrochemical Sciences (CES) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bledowski, M., Wang, L., Ramakrishnan, A. et al. TiO2-polyheptazine hybrid photoanodes: Effect of cocatalysts and external bias on visible light-driven water splitting. Journal of Materials Research 28, 411–417 (2013). https://doi.org/10.1557/jmr.2012.297
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.297