Abstract
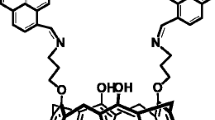
Polyaniline (PANI) chemically coated on poly(vinyl chloride) (PVC) membrane based on a neutral carrier 7,16-didecyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane (kryptofix 22 DD) as the active component has been developed for determination of pH values ranging from pH 0.1–1. The effect of experimental parameters such as membrane composition, nature and amount of plasticizer, lipophilic additives and thickness of PANI film on the potential response of the pH electrode was investigated. The electrode has an apparent Nernstian response slope of 54.5 ± 0.4 mV pH−1 (at 20°C). The equilibrium water content of the electrode was determined in pure water and NaCl solution (I = 0.1 mol Kg−1). The electrode had low electric resistance, good potential stability and reproducibility (±1.5 mV, n = 10). It has a rapid potential response to changes of pH (15 s). The excellent performance in terms of linearity, stability and fast response makes this device suitable for pH measurements in highly acidic media.

A new pH sensor electrode in highly acidic media (0.10–1.0 ) was constructed using polyaniline (PANI) chemically coated on PVC membrane based on a neutral carrier 7,16-didecyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane (kryptofix 22 DD) as the active component.






Similar content being viewed by others
References
Demirel A, Dogan A, Canel E, Memon S, Yilmaz M and Kilic E 2004 Talanta 62 123
Arvand M and Ghaiuri K 2009 Talanta 79 863
Erden S, Demirel A, Memon S, Yilmaz M, Canel E and Kilic E 2006 Sens. Actuators B 113 290
Coon R L and Lai N C 1967 J. Appl. Physiol. 40 625
Erne D, Ammann D and Simon W 1979 Chimia 33 88
Schulthess P, Shigo Y, Pham H V, Pretsch E and Simon W 1981 Anal. Chim. Acta 131 111
Oesch U, Brzozka Z, Xu A P, Rusterholz B, Suter G, Pham H V,Welti D H, Ammann D, Pretsch E and Simon W 1986 Anal. Chem. 2285 58
Yuan R, Qin Chai Y and Qin Yu R 1992 Analyst 117 1891
Fouskaki M, Gimisis T and Chaniotakis N 2002 Electroanalysis 14 593
Kuruoglu D, Canel E, Memon S, Yilmaz M and Kilic E 2003 Anal. Chem. 19 217
Liu X, Peng B, Liu F and Qin Y 2007 Sens. Actuators B 125 656
Cho D H, Chung K C and Park M Y 1998 Talanta 47 815
Cho D H, Chung K C, Jeong S S and Park M Y 2000 Talanta 51 761
Michalska A, Hulannicki A and Lewenstam A 1994 Analyst 119 417
Espedas-Torre C and MeyerhoffM E 1995 Anal. Chem. 67 3108
Nikolskii B P andMaterova E A 1985 Ion-Sel. Electrode Rev. 7 3
Pandey PC and Prakash R 1998 J. Electrochem. Soc. 145 4103
Lindfors T, Bobacka J, Lewenstam A and Ivaska A 1996 Analyst 121 1823
Bobacka J 1999 Anal. Chem. 71 4932
Papathanassious A N, Sakellis I, Grammatikakis J, Sakkopoulos S, Vitoratos E and Dalas E 2002 J. Phys. Appl. Phys. 85 35
Maikaj P, Dalas E, Vitoratos E and Sakkopoulos S 2006 J. Appl. Polym. Sci. 101 1853
Diaz A F, Cassillo J L, Logan J A and Lee W Y 1981 J. Electroanal. Chem. 129 115
Hamdani K and Cheng K L 1999 Microchem. J. 61 198
Han W S, Park M, Cho D, Hong T, Lee D, Park J and Chung K 2001 Anal. Sci. 17 727
Han W S, Chung K, Kim M, Ko H, Lee Y and Hong T 2004 Anal. Sci. 20 1419
Han W S, Park M, Chung K, Cho D and Hong T 2001 Talanta 54 153
Han W S, Park M, Chung K, Cho D and Hong T 2001 Electroanalysis 13 955
Zine N, Bausells J, Teixidor F, Vinas C, Masalles C, Samitier J and Errachid A 2006 Mater. Sci. Eng. C 26 99
Prasad G K, Radhakrishnan T P, Kumar D S and Krishn M G 2005 Sens. Actuators B 106 626
Xu K, Zhu L, Li J and Tang H 2006 Electrochim. Acta. 52 723
Sadek A Z, Wlodarski W, Kalantar-Zadeh K, Baker C and Kaner R B 2007 Sens. Actuators A 139 53
Shishkanova T V, Sapurina I, Stejskal J, Kral V and Volf R 2005 Anal. Chim. Acta 553 160
Stejskal J, Sapurina I, Prokes J and Zemck J 1999 Synth. Mat. 105 195
Stejskal J and Sapurina I 2005 Appl. Chem. 77 815
Stejskal J, Hlavata D, Holler P, Trchova P, Prokes M J and Sapurina I 2004 Polym. Int. 53 294
Ayad M M, Salahuddin N A, Alghaysh M O and Issa R M 2010 Curr. Appl. Phys. 10 235
Cosofret V, Erdosy M, Raleigh J, Johnson T, Neuman M and Buck R 1996 Talanta 43 143
E Bakker, P Buhlmann, and E Pretsch, Chem. Rev. 97 (1997), 3083
V V Egorov and Y V Sin’kevich, Talanta 48 (1999), 23
E Bakker, Anal. Chem. 69 (1997), 1061
A Radu, S Peper, E Bakker, and D Diamond, Electroanalysis 19 (2007), 144
Acknowledgement
The authors are thankful to the Post-Graduate Office of Guilan University for the support to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ANSARI, R., ARVAND, M. & HEYDARI, L. The behaviour of polyaniline-coated Poly(vinyl chloride) membrane based on 7, 16-didecyl-1, 4, 10, 13-tetraoxa-7, 16-diazacyclooctadecane for pH measurements in highly acidic media. J Chem Sci 126, 41–48 (2014). https://doi.org/10.1007/s12039-013-0554-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0554-z