Abstract
Objective
To compare the effect between intra-articular infiltration of low molecular weight (LMW-HA) and high molecular weight hyaluronic acid (HMW-HA) on the histopathological characteristics of the cartilage and disc of the temporomandibular joint (TMJ) osteoarthritis (OA) induced in rabbits.
Material and methods
An experimental study was conducted on 38 rabbit TMJs. The effect of different hyaluronic acids was compared at 30 and 135 days. Histopathological analysis was performed. Cartilage damage was assessed with the OARSI scale.
Results
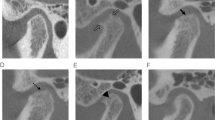
The severity of the induced OA according to OARSI was 3.4 degrees in the mandibular condyle (MC) and 3.2 in the mandibular fossa (MF); the articular disc (AD) presented disorganization of the collagen fibers, with randomly arranged hypertrophic chondrocytes. At 30 days, untreated TMJs worsened. TMJ treated with LMW-HA reduced its severity to 1.5 degrees in MC and 1.6 in MF, the AD presented histological aspects within normal limits. TMJ treated with HMW-HA presented 2.4 degrees in MC and 2.2 in MF, the AD maintained characteristics similar to the group with OA. At 135 days, all groups worsened.
Conclusion
Exogenous HA is effective in the management of TMJ-OA induced in rabbits, showing cartilage and articular disc repair at 30 days. The LMW-HA group had better effects on joint tissue than HMW-HA 30 days after treatment. However, at 135 days, both groups presented regression of joint tissue repair.
Clinical relevance
HA is effective in the anti-arthritic treatment of TMJ-OA induced in rabbits; LMW-HA shows better results in cartilage and articular disc repair than HMW-HA.




Similar content being viewed by others
References
Koszowska A, Hawranek R, Nowak J (2014) Osteoarthritis – a multifactorial issue. Rheumatology 52:319–325. https://doi.org/10.5114/reum.2014.46670
Cledes G, Felizardo R, Foucart JM, Carpentier P (2006) Validation of a chemical osteoarthritis model in rabbit temporomandibular joint: a compliment to biomechanical models. Int J Oral Maxillofac Surg 35:1026–1033. https://doi.org/10.1016/j.ijom.2006.05.003
Güler N, Kürkçü M, Duygu G, Çam B (2011) Sodium iodoacetate induced osteoarthrosis model in rabbit temporomandibular joint: CT and histological study (part I). Int J Oral Maxillofac Surg 40:1289–1295. https://doi.org/10.1016/j.ijom.2011.07.908
Dahl LB, Dahl IM, Engström-Laurent A, Granath K (1985) Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis 44:817–822. https://doi.org/10.1136/ard.44.12.817
Takahashi T, Tominaga K, Takano H, Ariyoshi W, Habu M, Fukuda J, Maeda H (2004) A decrease in the molecular weight of hyaluronic acid in synovial fluid from patients with temporomandibular disorders. J Oral Pathol Med 33:224–229. https://doi.org/10.1111/j.0904-2512.2004.00024.x
Balazs EA, Watson D, Duff IF, Roseman S (1967) Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum 10:357–376. https://doi.org/10.1002/art.1780100407
Brandt K, Smith G, Simon L (2000) Intraarticular injection of hyaluronan as treatment for knee osteoarthritis what is the evidence? Arthritis Rheum 43:1192–1203. https://doi.org/10.1002/1529-0131(200006)43:6<1192::AID-ANR2>3.0.CO;2-L
Mathiessen A, Conaghan PG (2017) Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 19:18. https://doi.org/10.1186/s13075-017-1229-9
Manfredini D, Rancitelli D, Ferronato G, Guarda-Nardini L (2012) Arthrocentesis with or without additional drugs in temporomandibular joint inflammatory-degenerative disease: comparison of six treatment protocols. J Oral Rehabil 39:245–251. https://doi.org/10.1111/j.1365-2842.2011.02265.x
Uchôa M, Constantino G (2012) Viscosupplementation. Rev Bras Ortop 47:160–164. https://doi.org/10.1016/S2255-4971(15)30080-X
Coronado L, Iturriaga V, Bornhardt T, Fuentes R (2015) Application protocol of hyaluronic acid on temporomandibular joint degenerative diseases. Av Odontoestomatol 31:77–84. https://doi.org/10.4321/S0213-12852015000200004
Sato S, Oguri S, Yamaguchi K, Kawamura H, Motegi K (2001) Pumping injection of sodium hyaluronate for patients with non-reducing disc displacement of the temporomandibular joint: two year follow-up. J Craniomaxillofac Surg 29:89–93. https://doi.org/10.1054/jcms.2000.0189
Poole R, Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, Matyas J, McDougall J, Pritzker K, Rudolphi K, van den Berg W, Yaksh T (2010) Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthr Cartil 18:S10–S16. https://doi.org/10.1016/j.joca.2010.05.027
Institute for Laboratory Animal Research (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington (DC)
Duygu G, Güler B, Cam M, Kürkcü (2011) The effects of high molecular weight hyaluronic acid (Hylan G-F 20) on experimentally induced temporomandibular joint osteoartrosis: part II. Int J Oral Maxillofac Surg 40:1406–1413. https://doi.org/10.1016/j.ijom.2011.07.909
Iturriaga V, Vásquez B, Veuthey C, del Sol C (2019) Temporomandibular joint arthrocentesis in a rabbit model: technique and recommendations in the study of temporomandibular disorders. Pol J Vet Sci 22:321–326. https://doi.org/10.24425/pjvs.2019.129223
Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP (2010) The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthr Cartil 18:S53–S65. https://doi.org/10.1016/j.joca.2010.05.029
Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14:13–29. https://doi.org/10.1016/j.joca.2005.07.014
Pritzker KP, Aigner T (2010) Terminology of osteoarthritis cartilage and bone histopathology - a proposal for a consensus. Osteoarthr Cartil 18:S7–S9. https://doi.org/10.1016/j.joca.2010.05.028
Aigner T, Cook JL, Gerwin N, Glasson SS, Laverty S, Little CB, McIlwraith W, Kraus VB (2010) Histopathology atlas of animal model systems - overview of guiding principles. Osteoarthr Cartil 18:S2–S6. https://doi.org/10.1016/j.joca.2010.07.013
Dinno A (2015) Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J 15:292–300. https://doi.org/10.1177/1536867X1501500117
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045. https://doi.org/10.1093/beheco/arh107
Poole AR, Rizkalla G, Ionescu M, Reiner A, Brooks E, Rorabeck C, Bourne R, Bogoch E (1993) Osteoarthritis in the human knee: a dynamic process of cartilage matrix degradation, synthesis and reorganization. Agents Actions Suppl 39:3–13. https://doi.org/10.1007/978-3-0348-7442-7_1
Chen YQ, Chou PL, Cheng CY, Chiang CC, Wei MT, Chuang CT, Chen YL, Chiou A (2012) Microrheology of human synovial fluid of arthritis patients studied by diffusing wave spectroscopy. J Biophotonics 5:777–7784. https://doi.org/10.1002/jbio.201100128
Alvarez C, Monasterio G, Cavalla F, Córdova LA, Hernández M, Heymann D, Garlet GP, Sorsa T, Pärnänen P, Lee HM, Golub LM, Vernal R, Kantarci A (2019) Osteoimmunology of oral and maxillofacial diseases: translational applications based on biological mechanisms. Front Immunol 10:1664. https://doi.org/10.3389/fimmu.2019.01664
Guidolin DD, Ronchetti IP, Lini E, Guerra D, Frizziero L (2001) Morphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500–730 kDa sodium hyaluronate (Hyalgan) and methylprednisolone acetate on primary osteoarthritis of the knee. Osteoarthr Cartil 9:371–381. https://doi.org/10.1053/joca.2000.0398
Dėdinaitė A, Wieland DCF, Bełdowski P, Claesson PM (2019) Biolubrication synergy: hyaluronan - phospholipid interactions at interfaces. Adv Colloid Interf Sci 274:102050. https://doi.org/10.1016/j.cis.2019.102050
Wang M, Liu C, Thormann E, Dėdinaitė A (2013) Hyaluronan and phospholipid association in biolubrication. Biomacromolecules 14:4198–4206. https://doi.org/10.1021/bm400947v
Xinmin Y, Jian H (2005) Treatment of temporomandibular joint osteoarthritis with viscosupplementation and arthrocentesis on rabbit model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:e35–e38. https://doi.org/10.1016/j.tripleo.2004.12.025
Neo H, Ishimaru J, Kurita K, Goss AN (1997) The effect of hyaluronic acid on experimental temporomandibular joint osteoarthrosis in the sheep. J Oral Maxillofac Surg 55:1114–1119. https://doi.org/10.1016/s0278-2391(97)90293-7
Moreland LW (2003) Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther 5:54–67. https://doi.org/10.1186/ar623
Iturriaga V, Vásquez B, Manterola C, del Sol M (2017) Role of hyaluronic acid in the homeostasis and therapeutics of temporomandibular joint osteoarthritis. Int J Morphol 35:901–907. https://doi.org/10.4067/S0717-95022017000300012
Iturriaga V, Bornhardt T, Manterola C, Brebi P (2017) Effect of hyaluronic acid in the regulation of inflammatory mediators in osteoarthritis of the temporomandibular joint: systematic review. Int J Oral Maxillofac Surg 46:590–595. https://doi.org/10.1016/j.ijom.2017.01.014
Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM (2006) High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr Cartil 14:1237–1247. https://doi.org/10.1016/j.joca.2006.05.009
Ghosh P, Guidolin D (2002) Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum 32:10–37. https://doi.org/10.1053/sarh.2002.33720
Wadhwa S, Kapila S (2008) TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ 72:930–947
Bergman AA, Heidger PM (1996) Histology. W.B. Saunders Company, Iowa City
Iturriaga V, Mena P, Oliveros R, Cerda C, Torres D, del Sol M (2018) Value of synovial fluid in the temporomandibular joint and its implications in articular pathology. Int J Morphol 36:297–302. https://doi.org/10.4067/S0717-95022018000100297
Hempfling H (2007) Intra-articular hyaluronic acid after knee arthroscopy: a two-year study. Knee Surg Sports Traumatol Arthrosc 15:537–546. https://doi.org/10.1007/s00167-006-0260-1
Bertolami CN, Gay T, Clark GT, Rendell J, Shetty V, Liu C, Swann DA (1993) Use of sodium hyaluronate in treating temporomandibular joint disorders: a randomized, double-blind, placebo-controlled clinical trial. J Oral Maxillofac Surg 51:232–242. https://doi.org/10.1016/s0278-2391(10)80163-6
Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M (2001) Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem 276:35111–35122. https://doi.org/10.1074/jbc.M103481200
Smith MM, Ghosh P (1987) The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int 7:113–122. https://doi.org/10.1007/BF00270463
Scott D, Coleman PJ, Mason RM, Levick JR (2000) Concentration dependence of interstitial flow buffering by hyaluronan in synovial joints. Microvasc Res 59:345–353. https://doi.org/10.1006/mvre.1999.2231
Coleman PJ, Scott D, Mason RM, Levick JR (2000) Role of hyaluronan chain length in buffering interstitial flow across synovium in rabbits. J Physiol 526:425–434. https://doi.org/10.1111/j.1469-7793.2000.00425.x
Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y (1998) Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol 61:125–135. https://doi.org/10.1679/aohc.61.125
Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA (2015) High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng 1:481–493. https://doi.org/10.1021/acsbiomaterials.5b00181
Bagga H, Burkhardt D, Sambrook P, March L (2006) Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol 33:946–950
Ghosh P, Read R, Numata Y, Smith S, Armstrong S, Wilson D (1993) The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. II. Cartilage composition and proteoglycan metabolism. Semin Arthritis Rheum 22:31–42. https://doi.org/10.1016/s0049-0172(10)80017-4
Guarda-Nardini L, Cadorin C, Frizziero A, Ferronato G, Manfredini D (2012) Comparison of 2 hyaluronic acid drugs for the treatment of temporomandibular joint osteoarthritis. J Oral Maxillofac Surg 70:2522–2530. https://doi.org/10.1016/j.joms.2012.07.020
Guarda-Nardini L, Rossi A, Arboretti R, Bonnini S, Stellini E, Manfredini D (2015) Single- or multiple-session viscosupplementation protocols for temporomandibular joint degenerative disorders: a randomized clinical trial. J Oral Rehabil 42:521–528. https://doi.org/10.1111/joor.12282
Acknowledgments
The authors appreciate the role of Prof. Adriana Vasconcellos for her professional support as a medical pathologist in this study.
Funding
The study was financed by Project DI20-0018 and the Temporomandibular Disorder and Orofacial Pain Program, Universidad de La Frontera, Chile. The funding bodies did not participate in the conception, design, or execution of the project that was carried out independently.
Author information
Authors and Affiliations
Contributions
All authors contributed with the conception and design of the study, drafting the article, critically revising the manuscript for important intellectual content, and giving final approval and agreeing to be accountable for all aspects of this work. Veronica Iturriaga contributed with the experimental procedures and data analysis and interpretation. Bélgica Vásquez contributed with data analysis and interpretation. Mariano del Sol contributed by providing study materials and data interpretation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Universidad de La Frontera (File No. 083/2016).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1078 kb).
Rights and permissions
About this article
Cite this article
Iturriaga, V., Vásquez, B., Bornhardt, T. et al. Effects of low and high molecular weight hyaluronic acid on the osteoarthritic temporomandibular joint in rabbit. Clin Oral Invest 25, 4507–4518 (2021). https://doi.org/10.1007/s00784-020-03763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03763-x