Abstract
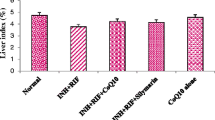
Isoniazid (INH) and rifampicin (RIF) are the first-line drugs for antituberculosis (anti-TB) chemotherapy. The levels of serum transaminases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] are abnormal in 27% of patients undergoing INH and RIF treatments and in 19% of patients undergoing treatment with INH alone. Cytochrome P450 2E1 (CYP2E1) metabolizes many toxic substrates, including ethanol, carbon tetrachloride, and INH, which ultimately results in liver injury. The objective of this study was to screen for CYP2E1 inhibitors in vitro and investigate whether the selected compound could prevent INH/RIF-induced hepatotoxicity in vivo. We screened 83 known compounds from food and herbal medicines as inhibitors of CYP2E1. The hepatotoxic dose of INH/RIF was 50/100 mg kg−1 day−1. Hepatotoxicity was assessed using galactose single-point (GSP) method (a quantitative measurement of liver function), histopathological examination of the liver, malondialdehyde (MDA) assay, and measurement of AST and ALT activities. Kaempferol inhibited CYP2E1 activity in mice by 0.31- to 0.48-fold (p < 0.005). Mice with INH/RIF-induced hepatotoxicity showed significantly abnormal serum levels of AST and ALT, and GSP value, and these values could be decreased by the administration of kaempferol (p < 0.005). Kaempferol significantly reduced the depletion of hepatic glutathione and prevented the increase in MDA formation in mice. Furthermore, kaempferol did not affect the anti-TB effects of INH/RIF. To our knowledge, this is the first report of kaempferol’s utility as an adjuvant for preventing CYP2E1-mediated hepatotoxicity induced by drugs such as INH and RIF.



Similar content being viewed by others
References
Zhang L, Wang Y, Zou P, Zhang H. Advances in clinical pharmacokinetics of herbal medicines. J US-China Med Sci. 2005;2:59–72.
Breinholt VM, Offord EA, Brouwer C, Nielsen SE, Brøsen K, Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chemical Toxicol. 2002;40:609–16.
Kane GC, Lipsky JJ. Drug–grapefruit juice interactions. Mayo Clin Proc. 2000;75:933–42.
Ono S, Hatanaka T, Hotta H, Satoh T, Gonzalez FJ, Tsutsui M. Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of In vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica. 1996;26:681–93.
Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30:576–81.
Gradolatto A, Canivenc-Lavier MC, Basly JP, Siess MH, Teyssier C. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug Metab Dispos. 2004;32:58–65.
Cermak R. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin Drug Metab Toxicol. 2008;4:17–35.
Wojnowski L, Kamdem LK. Clinical implications of CYP3A polymorphisms. Expert Opin Drug Metab Toxicol. 2006;2:171–82.
Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specific cities and tissue distribution of cytochrome P450 3A and P-glycoprotein. Implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–34.
Ueng YF, Shyu CC, Lin YL, Park SS, Liao JF, Chen CF. Effects of baicalein and wogonin on drug-metabolizing enzymes in C57BL/6J mice. Life Sci. 2000;67:2189–200.
Jang SI, Kim HJ, Hwang KM. Hepatoprotective effect of baicalin, a major flavone from Scutellaria radix, on acetaminophen-induced liver injury in mice. Immunopharmacol Immunotoxicol. 2003;25:585–94.
Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37:924–30.
Walubo A, Smith P, Folb PI. The role of oxygen free radicals in isoniazid-induced hepatotoxicity. Meth Find Exp Clin Pharmacol. 1998;20:649–55.
Yue J, Peng RX, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol Sin. 2004;25:699–704.
Nicod L, Viollon C, Regnier A, Jacqueson A, Richert L. Rifampicin and isoniazid increase acetaminophen and isoniazid cytotoxicity in human HepG2 hepatoma cells. Hum Exp Toxicol. 1997;16:28–34.
Tafazoli S, Mashregi M, O’Brien PJ. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol Appl Pharmacol. 2008;229:94–101.
Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–7.
Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, et al. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol. 2006;62:423–9.
Cederbaum AI. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J Gastroenterol Hepatol. 2006;21:S22–5.
Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC. Isoniazid and rifampicin induced oxidative hepatic injury protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19:517–24.
Gurumurthy P, Krishnamurthy M, Nazareth O. Lack of relationship between hepatic toxicity and acetylator phenotype and in South Indian patients during treatment with isoniazid for tuberculosis. Am Rev Respir Dis. 1984;129:58–61.
Mitchell JR, Thorgeisson UP, Black M, Timbrell JA, Snodgrass WR, Potter WZ, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydrazine metabolites. Clin Pharmacol Ther. 1975;18:70–9.
Timbrell JA, Mitchell JR, Snodgrass WR, Nelson SD. Isoniazid hepatotoxicity: the relationship between covalent binding and metabolism in vivo. J Pharmacol Exp Ther. 1980;213:364–9.
Tasduq SA, Kaiser P, Sharma SC, Johri RK. Potentiation of isonoazid-induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: a toxicity profile study. Hepatol Res. 2007;37:845–53.
Sarich TC, Adams SP, Petricca G, Wright JM. Inhibition of isoniazid-induced hepatotoxicity in rabbits by pretreatment with an amidase inhibitor. J Pharmacol Exp Ther. 1999;289:695–702.
Shen C, Meng Q, Zhang G, Hu W. Rifampicin exacerbates isoniazid-induced toxicity in human but not in rat hepatocytes in tissue-like cultures. Br J Pharmacol. 2008;153:784–91.
Yue J, Peng R. Does CYP2E1 play a major role in the aggravation of isoniazid toxicity by rifampicin in human hepatoctytes? Br J Pharmacol. 2009;157:331–3.
Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12.
Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006;45:117–26.
Ohkawa H, Ohish N, Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Tang HS, Hu OYP. Assessment of liver function using a novel galactose single point method. Digestion. 1992;52:222–31.
Hu OYP, Hu TM, Tang HS. Determination of galactose in human blood by high-performance liquid chromatograph: comparison with an enzymatic method and application to the pharmacokinetic study of galactose in patients with liver dysfunction. J Pharm Sci. 1995;84:231–5.
Hu OYP, Tang HS, Chang CL. Novel galactose single point method as a measure of residual liver function: example of cefoperazone kinetics in patients with liver cirrhosis. J Clin Pharmacol. 1995;35:250–8.
Hu OYP, Tang HS, Chang CL. The influence of chronic lobular hepatitis on pharmacokinetics of cefoperazone—a novel galactose single-point method as a measure of residual liver function. Biopharm Drug Dispos. 1994;15:563–76.
Cuatrecasas P, Segal S. Mammalian galactokinase. Developmental and adaptive characteristics in the rat liver. J Biol Chem. 1965;240:2382–88.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Kharasch ED, Thummel KE, Mhyre J, Lillibridge JH. Single-dose disulfiram inhibition of chlorzoxazone metabolism: a clinical probe for P450 2E1. Clin Pharmacol Ther. 1993;53:643–50.
Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5.
Oliveira EJ, Watson DG, Grant MH. Metabolism of quercetin and kaempferol by rat hepatocytes and the identification of flavonoid glycosides in human plasma. Xenobiotica. 2002;32:279–87.
Jeong HG. Inhibition of cytochrome P450 2E1 expression by oleanolic acid: hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett. 1999;105:215–22.
Acknowledgments
We are grateful to Drs. HS Lee and CB Hsieh from the Tri-Service General Hospital. The National Defense Medical Center supported this study.
Financial support
None.
Statement of Interest
No conflicts of interest are declared by all of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 339 kb)
Rights and permissions
About this article
Cite this article
Shih, TY., Young, TH., Lee, HS. et al. Protective Effects of Kaempferol on Isoniazid- and Rifampicin-Induced Hepatotoxicity. AAPS J 15, 753–762 (2013). https://doi.org/10.1208/s12248-013-9490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9490-6