Abstract
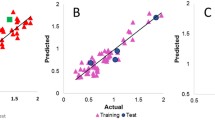
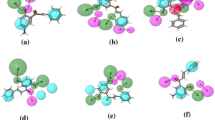
Chrysin is a derivative of flavonoid, a natural product commonly found in plants. It has been shown to afford a wide variety of pharmacological activities particularly anticancer properties. In this study, 21 chrysin derivatives with anticancer activities against human gastric adenocarcinoma (SGC-7901) and human colorectal adenocarcinoma (HT-29) cell lines were employed for quantitative structure–activity relationship (QSAR) investigation. Molecular structures were geometrically optimized at the B3LYP/6-311++g(d,p) level and their quantum chemical and molecular properties were obtained from Gaussian 09 and Dragon softwares, respectively. Significant descriptors for modeling the anticancer activities of SGC-7901 (i.e., SIC2, Mor11e, P2p, HTp, and R5e+) and HT-29 (i.e., L/Bw, BIC2, and Mor19p) cell lines were deduced from stepwise multiple linear regression (MLR) method. QSAR models were constructed using MLR and their predictivities were verified via internal (i.e., leave one-out cross-validation; LOO-CV) and external sets. The predictive performance was evaluated from their squared correlation coefficients (R 2 and Q 2) and root mean square error (RMSE). Results indicated good correlation between experimental and predicted anticancer activities as deduced from statistical parameters of internal and external sets as follows: R 2Tr = 0.8778, RMSETr = 0.0854, Q 2CV = 0.7315, RMSECV = 0.1375, Q 2Ext = 0.7324, and RMSEExt = 0.1168 for QSAR models of SGC-7901 while R 2Tr = 0.8201, RMSETr = 0.1293, Q 2CV = 0.6829, RMSECV = 0.1735, Q 2Ext = 0.8486, and RMSEExt = 0.1179 for QSAR models of HT-29. Furthermore, the obtained QSAR models provided pertinent insights on the structure–activity relationship of investigated compounds where molecular properties such as shape, electronegativities and polarizabilities were crucial for anticancer activity. The knowledge gained from the constructed QSAR models could serve as guidelines for the rational design of novel chrysin derivatives with potent anticancer activity.



Similar content being viewed by others
References
Bae Y, Lee S, Kim SH (2011) Chrysin suppresses mast cell-mediated allergic inflammation: involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol 254:56–64
Batra P, Sharma AK (2013) Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 3:439–459
Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109:3012–3043
Cushnie TP, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–356
DenningtonII R, Keith T, Millam J, Eppinnett K, Hovell WL, Gilliland R (2003) GaussView, Version 3.09. Semichem Inc, Shawnee Mission, KS
Drews J (2007) Drug discovery: a historical perspective. Science 287:1960–1964
Duchowicz PR, Bennardi DO, Bacelo DE, Bonifazi EL, Rios-Luci C, Padrón JM, Burton G, Misico RI (2014) QSAR on antiproliferative naphthoquinones based on a conformation-independent approach. Eur J Med Chem 77:176–184
Eriksson L, Johansson E (1996) Multivariate design and modeling in QSAR. Chemometr Intell Lab Syst 34:1–19
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Wallingford, Connecticut
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Ibrahim AK, Youssef AI, Arafa AS, Ahmed SA (2013) Anti-H5N1 virus flavonoids from Capparis sinaica Veill. Nat Prod Res 27:2149–2153
Ishihara M, Yokote Y, Sakagami H (2006) Quantitative structure-cytotoxicity relationship analysis of coumarin and its derivatives by semiempirical molecular orbital method. Anticancer Res 26:2883–2886
Ishihara M, Kawase M, Westman G, Samuelsson K, Motohashi N, Sakagami H (2007) Quantitative structure-cytotoxicity relationship analysis of phenoxazine derivatives by semiempirical molecular-orbital method. Anticancer Res 27:4053–4057
Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT (2005) The antitumor activities of flavonoids. In Vivo 19:895–909
Karelson M, Lobanov VS, Katritzky AR (1996) Quantum-chemical descriptors in QSAR/QSPR studies. Chem Rev 96:1027–1044
Khachatoorian R, Arumugaswami V, Raychaudhuri S, Yeh GK, Maloney EM, Wang J, Dasgupta A, French SW (2012) Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology 433:346–355
Kubo I, Kinst-Hori I, Chaudhuri SK, Kubo Y, Sánchez Y, Ogura T (2000) Flavonols from Heterotheca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg Med Chem 8:1749–1755
Mohammed HA, Ba LA, Burkholz T, Schumann E, Diesel B, Zapp J, Kiemer AK, Ries C, Hartmann RW, Hosny M, Jacob C (2011) Facile synthesis of chrysin-derivatives with promising activities as aromatase inhibitors. Nat Prod Commun 6:31–34
Nantasenamat C, Isarankura-Na-Ayudhya C, Naenna T, Prachayasittikul V (2007a) Quantitative structure-imprinting factor relationship of molecularly imprinted polymers. Biosens Bioelectron 22:3309–3317
Nantasenamat C, Isarankura-Na-Ayudhya C, Tansila N, Naenna T, Prachayasittikul V (2007b) Prediction of GFP spectral properties using artificial neural network. J Comput Chem 28:1275–1289
Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V (2010) Advances in computational methods to predict the biological activity of compounds. Expert Opin Drug Discov 5:633–654
Nantasenamat C, Worachartcheewan A, Prachayasittikul S, Isarankura-Na-Ayudhya C, Prachayasittikul V (2013a) QSAR modeling of aromatase inhibitory activity of 1-substituted 1,2,3-triazole analogs of letrozole. Eur J Med Chem 69:99–114
Nantasenamat C, Li H, Mandi P, Worachartcheewan A, Monnor T, Isarankura-Na-Ayudhya C, Prachayasittikul V (2013b) Exploring the chemical space of aromatase inhibitors. Mol Divers 17:661–677
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Parr RG, Szentpaly Lv, Liu S (1999) Electrophilicity Index. J Am Chem Soc 121:1922–1924
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K (2009) Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets 8:229–235
Sathiavelu J, Senapathy GJ, Devaraj R, Namasivayam N (2009) Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-induced toxicity in female albino rats. J Pharm Pharmacol 61:809–817
Serafini M, Peluso I, Raguzzini A (2010) Flavonoids as anti-inflammatory agents. Proc Nutr Soc 69:273–278
Sun LP, Chen AL, Hung HC, Chien YH, Huang JS, Huang CY, Chen YW, Chen CN (2012) Chrysin: a histone deacetylase 8 inhibitor with anticancer activity and a suitable candidate for the standardization of Chinese propolis. J Agric Food Chem 60:11748–11758
Takahashi T, Kokubo R, Sakaino M (2004) Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett Appl Microbiol 39:60–64
Thanikaivelan P, Subramanian V, Raghava Rao J, Unni Nair B (2000) Application of quantum chemical descriptor in quantitative structure activity and structure property relationship. Chem Phys Lett 323:59–70
Tropsha A, Gramatica P, Gombar VK (2003) The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb Sci 22:69–77
Wang J, Qiu J, Dong J, Li H, Luo M, Dai X, Zhang Y, Leng B, Niu X, Zhao S, Deng X (2011) Chrysin protects mice from Staphylococcus aureus pneumonia. J Appl Microbiol 111:1551–1558
Witten IH, Frank E, Hall MA (2011) Data mining: practical machine learning tools and techniques, 3rd edn. Morgan Kaufmann, San Francisco
Woo KJ, Jeong YJ, Park JW, Kwon TK (2004) Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun 325:1215–1222
Worachartcheewan A, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V (2013a) QSAR study of amidino bis-benzimidazole derivatives as potent anti-malarial agents against Plasmodium falciparum. Chem Pap 67:1462–1473
Worachartcheewan A, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V (2013b) Predicting antimicrobial activities of benzimidazole derivatives. Med Chem Res 22:5418–5430
Worachartcheewan A, Nantasenamat C, Owasirikul W, Monnor T, Naruepantawart O, Janyapaisarn S, Prachayasittikul S, Prachayasittikul V (2014) Insights into antioxidant activity of 1-adamantylthiopyridine analogs using multiple linear regression. Eur J Med Chem 73:258–264
Zhang S, Yang X, Morris ME (2004a) Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol 65:1208–1216
Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y (2004b) Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem 12:6097–6105
Zheng X, Meng WD, Xu YY, Cao JG, Qing FL (2003) Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett 13:881–884
Zheng X, Zhao FF, Liu YM, Yao X, Zheng ZT, Luo X, Liao DF (2010) Synthesis and preliminary biological evaluation of chrysin derivatives as potential anticancer drugs. Med Chem 6:6–8
Acknowledgments
This research project is supported by the annual budget grant of Mahidol University (B.E. 2556–2558). A. W. is thankful for Mahidol University Talent Management Program. Partial support is gratefully acknowledged from Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Worachartcheewan, A., Nantasenamat, C., Isarankura-Na-Ayudhya, C. et al. Probing the origins of anticancer activity of chrysin derivatives. Med Chem Res 24, 1884–1892 (2015). https://doi.org/10.1007/s00044-014-1260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1260-1