Abstract
We have developed a crown ether based selective colorimetric sensing scheme for the determination of Pb(II) ion by using gold nanoparticles modified with dithiocarbamate derivative of 4′-aminobenzo-18-crown-6 that acts as a colorimetric probe. Monodisperse Au-NPs were prepared by reacting 4′-aminobenzo-18-crown-6 with carbon disulfide to generate the dithiocarbamate ligand which was then added to the Au-NPs to form a supramolecular assembly on their surface. The Au-NPs modified in this way undergo aggregation in the presence of Pb(II) ions, and this causes the color to change from red to blue. The Pb(II)-induced aggregation can be monitored by using UV-visible spectrometry and even with the bare eye. The absorbance ratio (A650nm/A520nm) is linearly related to the concentration of Pb(II) in the 0.1 to 75 μM range (with a correlation coefficient of 0.9957), and the detection limit is 50 nM which is lower than the allowable level (75 nM) as defined by the US EPA. The method was successfully applied to the determination of Pb(II) in spiked water samples.

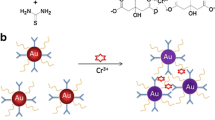
Schematic representation of Pb2+ ion-induced DTC-CE-Au NPs aggregation via sandwich complex formation.







Similar content being viewed by others
References
Global health risks: Mortality and burden of disease attributable to selected major risks. Geneva, World Health Organization, 2009 (http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf, accessed 20 December 2010)
Exposure to lead: A major public health concern. Geneva, World Health Organization, 2010 (http://www.who.int/ipcs/features/lead..pdf, accessed 20 December 2010).
Casas JS, Sordo J (2006) Lead: chemistry, analytical aspects, environmental impact and health effects. Elsevier, Amsterdam
Lin YW, Huang CC, Chang HT (2011) Gold nanoparticle probes for the detection of mercury, lead and copper ions. Analyst 136:863–871
Chai F, Wang C, Wang T, Li L, Su Z (2010) Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl Mater Interfaces 2:1466–1470
Beqa L, Singh AK, Khan SA, Senapati D, Arumugam SR, Ray PC (2011) Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl Mater Interfaces 3:668–673
Liu J, Lu Y (2004) Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J Am Chem Soc 126:12298–12305
Guan J, Jiang L, Zhao LL, Li J, Yang WS (2008) pH-dependent response of citrate capped Au nanoparticle to Pb2+ ion. Colloid Surf A 325:194–197
Wei H, Li BL, Li J, Dong SJ, Wang EK (2008) DNAzyme-based colorimetric sensing of lead (Pb2+) using unmodified gold nanoparticle probes. Nanotechnology 19:95501
Ferhan AR, Guo L, Zhou X, Chen P, Hong S, Kim DH (2013) Solid-phase colorimetric sensor based on gold nanoparticle-loaded polymer brushes: lead detection as a case study. Anal Chem 85:4094–4099
Gokel GW, Leevy WM, Michelle EW (2004) Crown ethers: sensors for ions and molecular scaffolds for materials and biological models. Chem Rev 104:2723–2750
Ijeri VS, Srivastava AK (2002) Complexation of macrocyclic compounds with metal ions: 1. Cd(II), Pb(II), Co(II), Mn(II), and Ag(I) ions in 40 vol ethanol + water medium. J Chem Eng Data 47:346–350
Chen CY, Cheng CT, Lai CW, Wu PW, Wu KC, Chou PT, Chou YH, Chiu HT (2006) Potassium ion recognition by 15-crown-5 functionalized CdSe/ZnS quantum dots in H2O. Chem Commun 42:263–265
Ho ML, Hsieh JM, Lai CW, Peng HC, Kang CC, Wu IC, Lai CH, Chen YC, Chou PT (2009) 15-crown-5 functionalized Au nanoparticles synthesized via single molecule exchange on silica nanoparticles: its application to probe 15-crown-5/K+/15-crown-5 “sandwiches” as linking mechanisms. J Phys Chem C 113:1686–1693
Patel G, Kumar A, Pal U, Menon S (2009) Potassium ion recognition by facile dithiocarbamate assembly of benzo-15-crown-5–gold nanoparticles. Chem Commun 45:1849–1851
Lin SY, Liu SW, Lin CM, Chen CH (2002) Recognition of potassium ion in water by 15-crown-5 functionalized gold nanoparticles. Anal Chem 74:330–335
Lin SY, Chen CH, Lin MC, Hsu HF (2005) A cooperative effect of bifunctionalized nanoparticles on recognition: sensing alkali ions by crown and carboxylate moieties in aqueous media. Anal Chem 77:4821–4828
Velu R, Ramakrishnan VT, Ramamurthy P (2010) Colorimetric and fluorometric chemosensors for selective signaling toward Ca2+ and Mg2+ by aza-crown ether acridinedione-functionalized gold nanoparticles. Tetrahedron Lett 51:4331–4335
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Anandhakumar S, Mathiyarasu J (2013) Detection of lead (II) using an glassy carbon electrode modified with Nafion, carbon nanotubes and benzo-18-crown-6. Microchim Acta 180:1065–1071
Späth A, König B (2010) Molecular recognition of organic ammonium ions in solution using synthetic receptors. Beilstein J Org Chem 6:1–111
Kuang H, Chen W, Yan W, Xu L, Zhu Y, Liu L, Chu H, Peng C, Wang L, Kotov NA, Xu C (2011) Crown ether assembly of gold nanoparticles: melamine sensor. Biosens Bioelectron 26:2032–2037
Flink S, Veggel FCJM, Reinhoudt DN (1999) Recognition of cations by self-assembled monolayers of crown ethers. J Phys Chem B 103:6515–6520
Pedersen CJ (1988) The discovery of crown ethers (Noble lecture). Angew Chem Int Ed 27:1021–1027
Jackson ML (1985) In soil chemical analysis. Parallel press, Wisconsin
Wei F, Lam R, Cheng S, Lu S, Ho D, Li N (2010) Rapid detection of melamine in whole milk mediated by unmodified gold nanoparticles. Appl Phys Lett 96:133702–133703
Lin CY, Yu CJ, Lin YH, Tseng WL (2010) Colorimetric sensing of silver (I) and mercury (II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal Chem 82:6830–6837
Miao XM, Ling LS, Shuai XT (2012) Detection of Pb2+ at attomole levels by using dynamic light scattering and unmodified gold nanoparticles. Anal Biochem 421:582–586
Jiang C, Ma M, Wang Y (2012) Using gallic acid-modified gold nanoassemblies to detect the Pb2+ of tea. Anal Methods 4:3570–3574
Zhang Y, Leng Y, Miao L, Xin J, Wu A (2013) The colorimetric detection of Pb2+ by using sodium thiosulfate and hexadecyl trimethyl ammonium bromide modified gold nanoparticles. Dalton Trans 42:5485–5490
Guo Y, Wang Z, Qu W, Shao H, Jiang X (2011) Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron 26:4064–4069
Cao H, Wei M, Chen Z, Huang Y (2013) Dithiocarbamate-capped silver nanoparticles as a resonance light scattering probe for simultaneous detection of lead (II) ions and cysteine. Analyst 138:2420–2426
Qi L, Shang Y, Wu F (2012) Colorimetric detection of lead (II) based on silver nanoparticles capped with iminodiacetic acid. Microchim Acta 178:221–227
Zhang L, Yao Y, Shan J, Li H (2011) Lead (II) ion detection in surface water with pM sensitivity using aza-crown-ether-modified silver nanoparticles via dynamic light scattering. Nanotechnology 27:275504
Acknowledgment
We thank the Director, SVNIT for providing all the facilities to carry out this work. We also thank Department of Science and Technology, India for providing UV-visible spectrophotometer under Fast-track Young Scientist Program (SR/FT/CS-54/2010). We would like to thank Mr. Vikas Patel, SICART, V. V. Nagar, Anand for their assistance in TEM data. We thank Prof. Z.V.P. Murthy and Mr. Chetan Patel, Chemical Engineering Department, SVNIT, Surat, India for providing DLS measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 901 kb)
Rights and permissions
About this article
Cite this article
Mehta, V.N., Solanki, J.N. & Kailasa, S.K. Selective visual detection of Pb(II) ion via gold nanoparticles coated with a dithiocarbamate-modified 4′-aminobenzo-18-crown-6. Microchim Acta 181, 1905–1915 (2014). https://doi.org/10.1007/s00604-014-1287-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1287-5