Abstract
The Food and Drug Administration (FDA) approved the first SARS-CoV-2 mRNA vaccine (Pfizer-BioNTech) in December 2020. New adverse events have emerged since these vaccines have reached market. Although no clear association between messenger ribonucleic acid (mRNA) vaccines and autoimmunity has emerged, the significance of such an association warrants further exploration. After obtaining consent, a standardized survey on baseline characteristics and other relevant variables was conducted on unvaccinated individuals who were scheduled for vaccination and had not previously contracted COVID-19. Blood samples were collected from participants prior to the first dose, prior to the second dose, and 1 month after the second dose. All collected samples were tested for antinuclear antibody (ANA) titers using indirect immunofluorescence microscopy kits, and antiphospholipid (APS) immunoglobulin M (IgM) and immunoglobulin G (IgG) levels using an enzyme-linked immunoassay (ELISA) technique. ANA titers were positive for 9 participants out of 101 (8.9%) in the first pre-vaccination draw. For the second draw, the number of participants testing positive for ANA decreased to 5 (5%). For the last draw, 6 (5.9%) participants tested positive for ANA titers. One participant tested positive for APS IgM at the first pre-vaccination draw, 2 tested positive at the second draw, and 2 at the third draw. As for APS IgG titers, all participants tested negative in the three draws. McNemar’s test for two dependent categorical outcomes was conducted on all variables and did not show a statistical significance. The McNemar test of these two composite variables (i.e., ANA/APS, first draw vs. ANA/APS, second and third draws) did not show statistical significance. The 2-sided exact significance of the McNemar test was 1.0. The Friedman test also showed no significance (p = 0.459). No association was found between BNT162b2 vaccine administration and changes in APS and ANA titers. The benefits of the BNT162b2 vaccine significantly outweigh any possible risk of autoimmune dysregulation considering the current evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The focus of vaccine development has recently shifted from traditional methods such as live attenuated and protein subunit vaccines to nucleic acid vaccines. Nucleic acid vaccines are hypothesized to be more favorable for several considerations. For instance, nucleic acid vaccines are capable of triggering both an antibody-mediated and a cell-mediated immune response [1]. In addition, nucleic acid vaccines are expected to offer the advantage of lower cost and a simpler approach to mass production [1]. Also, nucleic acid vaccines can be considered a platform amenable to rapid changes since these vaccines can be easily modified to target new pathogens and new variants [1]. Despite promising preliminary results [2, 3], no such vaccine was successful in reaching the market prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. The US Food and Drug Administration (FDA) issued the first emergency use authorization on December 11, 2020, for the use of a messenger RNA (mRNA) vaccine (BNT162b2) against SARS-CoV-2 produced by Pfizer-BioNTech [4]. The initial report by the Advisory Committee on Immunization Practices concluded that the vaccine is safe for use with an acceptable range of side effects [4].
Although initial trials have confirmed the safety of the vaccine [5], the risk of emerging adverse events cannot be discounted, especially given the relatively short surveillance period prior to FDA emergency use authorization. Moreover, older vaccines were linked in separate reports to the occurrence of several autoimmune disorders following vaccination [6]. Systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, and Hashimoto thyroiditis have been observed following the administration of the HBV vaccine[7,8,9,10,11]. In addition, reactive arthritis, polyarteritis nodosa, and Guillain-Barré syndrome have been observed after the influenza vaccination [8, 12]. Similar autoimmune syndromes following other vaccines were also reported [13]. In addition, the incidence and prevalence of autoimmune disorders are increasing worldwide [14], and experts may be inclined to label vaccines among the usual suspects [13, 15]. Several mechanisms have been hypothesized to underlie the autoimmune dysfunction following vaccine administration. One hypothesis suggests that the immune system is exposed to a non-self-antigen—this antigen being vaccine component or an incidental superimposed infection triggers an autoimmune reaction given that the individual is genetically predisposed [16, 17]. The reaction is the result of similar molecular structures between a given agent and self-antigens, thus eliciting a faulty immune reaction against self-antigens. This phenomenon is referred to as molecular mimicry [18, 19]. Other theories linked immune dysregulation following vaccine administration to the release of cytokines, activation of certain immune receptors, or the exposure of sequestered self-antigens[13, 20,21,22]. However, the exact underlying mechanism and risk factors of autoimmunity following vaccination are still not well defined [19].
Vaccination seems to be the most effective option to end or control the ongoing pandemic. This implies a wide-scale use of mRNA vaccines, among other types. Recently, various nations have allowed access to booster doses of the vaccines, with some countries exploring the option of even more doses [23]. Based on the literature presented above, we suggest that an association between the new SARS-CoV-2 vaccine and autoimmune antibodies—ANA and APS antibodies specifically—is worth exploring. We believe that the wide-scale use of these vaccines and booster campaigns makes it imperative to examine such correlations in an expedited manner.
In this study, we try to determine if a correlation between administration of the Pfizer-BioNTech SARS-CoV-2 vaccine (BNT162b2) and an elevation in autoimmune antibodies—specifically the antinuclear antibody (ANA) titers and antiphospholipid (APS) immunoglobulin M (IgM) and immunoglobulin G (IgG) levels—exists. We also aim to investigate the side effects of the vaccine and any possible correlation with the autoimmune antibodies under investigation.
Materials and methods
Data sources
Investigators randomly contacted consenting unvaccinated individuals who were scheduled through the Ministry of Health platform to take the vaccine. Demographic and baseline characteristics, past medical history, medication profile, and family history for autoimmune diseases were collected from participants through phone calls via a standardized survey prepared by the investigators. Information provided in this survey was used to determine if the participant fits the inclusion/exclusion criteria.
The participants were surveyed on their family history of autoimmune disorders and autoinflammatory disorders with a particular focus on rheumatoid arthritis, Crohn’s disease, ulcerative colitis, systemic lupus erythematous, psoriasis, familial Mediterranean fever, multiple sclerosis, type 1 diabetes mellitus, Guillain–Barre syndrome, Graves’ disease, Hashimoto’s thyroiditis, myasthenia gravis, and vasculitis. Participants were also surveyed for any allergies and for exposure to any medication that might interfere with their immune response to the vaccine. Individuals that were receiving immune modulators or immunosuppressive medications and people living with HIV were considered immunocompromised and thus excluded from the study. Individuals receiving inhaled corticosteroids were not considered immunocompromised and thus included in this study.
The participants were also surveyed on their history of COVID-19 infection. Participants who reported a previous COVID-19 infection were not included in this study. COVID-19 infection was also assessed in follow-up surveys and participants reporting COVID-19 infection were excluded. However, COVID-19 infection was not confirmed by PCR or antibody testing.
Follow-up surveys were conducted after the first and second shot of the vaccine. The surveys mainly focused on the side effects experienced by the participants following the shots.
Inclusion criteria
Individuals older than 18 years old or younger than 65 years old were included. Individuals who did not receive any COVID-19 vaccine and have never tested positive for COVID-19 by PCR or serology were included. Individuals with no previous history of immunocompromising condition or currently taking any immunosuppressive medication were included. Also, individuals who were not receiving any medication that is intended to prevent COVID-19 were included.
Exclusion criteria
Individuals younger than 18 years old or older than 65 years were excluded. In addition, individuals that had previously received any COVID-19 vaccine or had previously tested positive for COVID-19 either by PCR or serology were excluded. Pregnant or lactating women, immunocompromised individuals, and patients taking any medication intended to prevent COVID-19 were also excluded.
Blood sample collection
The first blood sample was obtained on the day of vaccination prior to receiving the first BNT162b2 dose. A second blood sample was then obtained in a similar fashion before the second dose of the vaccine 3–4 weeks later (mean: 21.4 ± 1.78 days). A third and final sample was obtained 1 month (mean: 33 ± 3.97 days) after the second vaccine dose. All 3 collected samples were tested for ANA titers using indirect immunofluorescence microscopy kits (EUROIMMUN; Lübeck, Germany), and APS IgM and IgG levels using an ELISA technique (ORGENTIC; Mainz, Germany) (Fig. 1).
Blood draws and laboratory analysis (created with Biorender.com)
For ANA levels, an indirect immunofluorescence microscopy assay (EUROIMMUN) was used. The serum was collected from blood samples and serially diluted, and then, IgG ANA antibodies were added. Fluoresceinated anti-IgG antibodies were added after the slide was washed and then viewed under fluorescent microscopy after being washed again (Fig. 2). The results of the test were reported as a composite of the dilution factor of the participant serum and the pattern of binding of antibodies. A dilution factor of 1:100 and above was considered positive [24, 25].
ANA titers and pattern analysis (created with Biorender.com)
For APS levels, a quantitative APS ELISA test (ORGENTIC) was used. The antiphospholipid screening kit detects four antibodies typically associated with antiphospholipid syndrome. The antibodies are specific to cardiolipin, phosphatidic acid, phosphatidylinositol, and phosphatidylserine. The kit detects IgG and IgM antibodies targeted against these four antigens. The results of this test can range from 0 to 100 IgG phospholipid units (GPL-U)/mL for IgG titers and 0–100 IgM phospholipid units (MPL-U)/mL for IgM titers. The cutoff was 10 GPL-U/mL for IgG titers and 10 MPL-U/mL for IgM titers as specified by the manufacturer’s protocol [26]. The test is sensitive to various APS antibodies, but does not distinguish between the different types [26].
Statistical analysis
Normality and descriptive statistics were conducted. Means and standard deviations were used to report continuous variables, while frequencies and percentages were used to report categorical variables. McNemar’s test for 2 dependent categorical outcomes was used to compare the results of our outcomes of interest at different time points (ANA and APS levels between draw 1 and draw 2, ANA and APS levels between draw 2 and draw 3). Additionally, the Friedman test, used to compare K dependent categorical outcomes, was used to confirm the results of the McNemar test. All statistical analyses were conducted using IBM SPSS version 28.0, and the alpha level was set at < 0.05 for statistical significance.
Ethical considerations.
The study was approved by the Lebanese American University institutional review board (LAU-IRB) (IRB# LAUMCRH.JM2.8/Feb/2021). Both verbal and written consents were secured from all participants. All personal and medical information of the participants was kept confidential. Participants with high ANA or APS titers were informed of the result and referred to a specialist. The initial survey and follow-up on vaccine side effects were conducted by phone to adhere with quarantine guidelines and ensure the safety of participants.
Results
Baseline characteristics
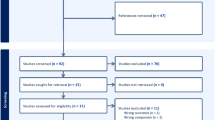
Of the 113 participants initially recruited, 12 were lost to follow-up or withdrew from the study. The final analysis was conducted on the remaining 101 participants. Baseline characteristics of the participants are reported in Table 1. Males constituted 43.6% of the sample, whereas female participants constituted 56.4%. The mean age was 37.6 years (SD = 11.9), with 28 participants between the age of 18 and 26 years, 20 participants between the age of 27 and 35 years, 22 participants between the age of 36 and 44, 16 participants between the age of 45 and 53 years, and 15 participants between the age of 54 and 62. More than 50% of the participants had an abnormal body mass index (BMI), whereby three individuals (3%) were underweight, 29 individuals (28.7%) were overweight, and 20 individuals (19.8%) were obese. Forty-seven participants (46.5%) reported a previously diagnosed health condition. The total number of individuals with allergies amounted to 30 individuals (29.7%) with 10 individuals (9.9%) receiving treatment for their allergies. The total number of participants with a family history of autoimmune disorders was 18 individuals (17.8%).
Side effects
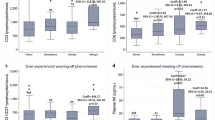
Following the first dose, 59 individuals (58.4%) reported side effects and 47 individuals (46.5%) reported side effects following the second dose of the vaccine. The side effects included fever, fatigue, myalgias, arthralgias, throat pain, diarrhea, abdominal pain, vomiting, pain at injection site, flu-like symptoms, rash, dizziness, headache, and lymphadenopathy. Five participants (5%) reported gastrointestinal side effects following the first dose, while none of the participants reported any gastrointestinal side effects following the second dose. As for pain at the injection site, 38 participants (37.6%) reported pain after the first dose, while only 14 participants (13.9%) reported pain following the second dose. Neurological symptoms, including headache and dizziness, were reported in 11 participants (10.9%) following the first dose and nine participants (8.9%) following the second dose. Six participants (5.9%) reported fever following the first dose, and 22 participants (21.8%) reported fever following the second dose. Also, 10 participants (9.9%) reported fatigue following the first dose, and 28 participants (27.7%) reported fatigue following the second dose. Table 2 illustrates the individual percentages of the side effects reported by the participants.
ANA and APS titers
Of the blood samples related to the first draw, i.e., prior to taking any dose of the vaccine, ANA titers were positive for 9 participants out of 101 (8.9%). For the second draw, prior to the second dose of the vaccine, the number of participants testing positive for ANA was only 5 (5%). For the last draw, 33 days after the second dose, 6 (5.9%) participants tested positive for ANA titers. The ANA positivity of the participants was variable in terms of titers and pattern (nuclear patterns including homogeneous, coarse speckled, fine speckled, centromere, and nucleolar and cytoplasmic patterns including diffuse and fine speckled) [27]. In addition, 3 participants consistently tested positive for ANA in all three samples. One participant tested positive for APS IgM at the first draw, 2 tested positive at the second draw, and 2 tested positive at the third draw. Only 1 participant tested positive for APS IgM in all 3 draws, with increasing titers with each draw (17 MPL-U/mL, 40 MPL-U/mL, and 60 MPL-U/mL in draw 1, draw 2, and draw 3 respectively). As for APS IgG titers, all participants tested negative in the three draws.
Statistical model and analysis
The McNemar test for two dependent categorical outcomes was conducted on all variables and did not show a statistical significance. For ANA titers compared in the first draw vs second draw, first draw vs third draw, and second draw vs third draw, the 2-sided exact significance was 0.727, 0.453, and 1.0 respectively. As for APS IgM titers in the first draw vs second draw, first draw vs third draw, and second draw vs third draw, the 2-sided exact significance was 1.0 for all combinations. Regarding the ANA and APS (IgM and IgG) titers compared in the first draw vs second draw and first draw vs third draw, the 2-sided exact significance was 1.0 and 0.727 respectively. Then, two composite variables were created. The first variable combined ANA and APS positivity at baseline (first draw); the second variable was a combination of ANA and APS positivity at the second and third blood draws. The McNemar test of these two composite variables (i.e., ANA/APS, first draw vs. ANA/APS, second and third draws) did not show statistical significance. The 2-sided exact significance of the McNemar test was 1.0. The Friedman test also showed no significance (p = 0.459).
Discussion
The results of this study do not reveal any association between the investigated autoimmune markers (ANA and APS) and the BNT162b2 mRNA vaccine. The McNemar test of the composite variables, ANA and APS at baseline versus ANA and APS in the second and third draws combined, was not significant (2-sided exact significance = 1.0). The McNemar test for ANA and APS (IgM and IgG) titers compared in the first draw vs second draw, first draw vs third draw, and second draw vs third draw variables was also not significant (Table 3). In contrast, numerous reports from the literature have documented a rise in autoantibodies and the emergence of autoimmune diseases following vaccination with traditional vaccines [28,29,30]. Human papillomavirus (HPV), influenza, and hepatitis B (HBV) vaccines were among the most studied in the literature. Two case series reported an association between the HPV vaccine and autoimmune disorders, mainly systemic lupus erythematous and dysautonomia [29, 30]. Furthermore, the HBV vaccine has been linked to the development of autoimmune conditions such as Guillain–Barre Syndrome (GBS) in rare cases[31]. In addition, the influenza vaccine was found to increase the levels of autoantibodies in some individuals with already elevated titers and may lead to the emergence of new antibodies in healthy individuals [28]. However, the influenza vaccine was not found to have an overall effect of increasing autoantibody levels within a given population [28].
Moreover, a recent meta-analysis found no correlation between the HPV vaccine and autoimmunity [32]. Also, two other cohort studies found no association between HPV vaccination and autoimmune disorders [33, 34]. However, an association between GBS and the HBV vaccine was confirmed in one of the studies [34]. The design, outcome, and vaccine of interest of these studies [28, 33, 34] do not align with the components of this study. Nevertheless, the findings of this study along with the aforementioned studies [28, 33, 34] do not suggest a strong association between vaccination in general and autoimmune dysregulation. The rarity of large studies supporting a causal relationship between vaccines and autoimmune dysfunction can be explained by several points. First, the incidence of autoimmune disorders triggered by vaccines is very low, such that it does not exceed the incidence of autoimmune disorders in the general population [35]. Second, the autoimmune manifestations following vaccination are not predictable except in select cases—HBV vaccine and GBS [34]—which makes studying such phenomena extremely challenging [36]. Third, even if autoimmune disorders do emerge following vaccination, the trigger of such dysregulation is subject to doubt and is usually attributed to more common etiologies such as infections [35]. Thus, it is important to emphasize that a lack of statistically significant causal relationship in large epidemiological studies does not eliminate the possibility that a causal relationship exists in individual cases.
In our study, there was no clear pattern of rising levels of ANA and APS titers, except for one female participant who had a significant increase in APS IgM levels after each dose (17 MPL-U/mL, 40 MPL-U/mL, and 60 MPL-U/mL in draw 1, draw 2, and draw 3 respectively). This patient did not report any side effects aside from transient fatigue and malaise. However, an isolated case of increasing APS IgM is not sufficient to draw associations between the BNT162b2 vaccine and autoimmune markers. It is possible that other external chronologic factors contributed to this rise. Also, it is imperative to note that antibody panel results (i.e., laboratory criteria) should be correlated with clinical criteria of antiphospholipid syndrome to confirm a diagnosis of antiphospholipid syndrome and such antibodies are commonly found detected as incidental findings [37]. Importantly, no individuals experienced seroconversion or de novo production of IgG APS, which is consistent with a previous small study on mRNA vaccines. The lack of seroconversion may in fact allude to the transient nature of autoimmune marker changes [38].
However, a few selected cases reported the development of specific antibodies following vaccination with SARS-CoV-2 mRNA vaccines. For instance, one case reported the formation of anti-glomerular basement membrane nephritis with IgA nephritis following the second dose of the mRNA-1273 SARS-CoV-2 vaccine in a previously healthy female patient [39]. Other cases included antineutrophil cytoplasmic autoantibody (ANCA) glomerulonephritis and anti-glomerular basement membrane nephritis following the second dose of Moderna SARS-CoV-2 mRNA vaccine [40, 41]. Until the time of writing, only isolated cases of autoantibody formation following SARS-CoV-2 mRNA vaccination were reported in the literature [39,40,41]. Such reports do not necessarily provide proof of a correlation between autoantibody formation and mRNA vaccines and large cohort studies are needed to investigate such correlations, if any. Moreover, a prospective cohort study from Germany on different types of SARS-CoV-2 vaccines including homologous and heterologous vaccine type administration did not find a correlation between SARS-CoV-2 vaccines and autoantibodies associated with common autoimmune disorders [42]. The study included participants receiving mRNA, vector type, and two heterologous shots of SARS-CoV-2 vaccines and did not find any association between these vaccines and the new-onset formation of autoantibodies commonly associated with lupus erythematosus, rheumatoid arthritis, celiac disease, and antiphospholipid syndrome [42]. The small sample size and the observational nature of the study [42] limit the ability to support strong conclusions; thus, larger cohort studies are needed to better solidify the lack of correlation between autoantibodies and SARS-CoV-2 vaccines.
On the other hand, the association between vaccines and autoimmune diseases is hard to quantify. The absence of an established association between vaccines and autoimmunity decreases the chances of such conditions being attributed to the vaccine of interest. It is possible that autoimmune conditions arising after vaccination could be subacute or have a delayed presentation [43]. Thus, in the search for such conditions, investigators should consider the wide variety of possible autoimmune conditions and do so over a generous timeline. However, the complexity of this theory makes it difficult to study on a small sample of patients. Moreover, it is important to examine the underlying mechanisms suggestive of such an association. mRNA vaccines have the potential to induce potent type I interferon (IFN) responses [44, 45] which have been linked with inflammation and potentially immune dysregulation [20, 21]. Yet, a clear association between mRNA vaccines and immune dysregulation needs to be established before delving further with such theories. At the time of writing, none of the participants of this study has reported developing any acute or subacute immune condition after the vaccine.
Vaccines aside, COVID-19 infection itself has been linked to immunologic dysregulation manifested as a rise in ANA and antiphospholipid (APL) antibody levels [18]. The severity of COVID-19 has been associated with the rise of concentration of these antibodies [18]. The mechanism of action of mRNA vaccines is based on producing epitopes also represented on pathogens, thus eliciting an immune response against them [46]. Like the viral infection itself, mRNA vaccines may cause immune cells to mistakenly produce antibodies against self-antigens [18]. Other possible mechanisms of autoimmunity have been proposed, such as the binding of RNA molecules to Toll-like receptors in endosomes and retinoic acid inducible gene-I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5) in the cytosol [22]. Both mechanisms lead to inflammatory cascades involving type I IFN and the nuclear translocation of the transcription factor nuclear factor (NF)-kB [22]. However, in the context of autoimmune changes, it is crucial to distinguish any associations established with the virus itself versus those established with the vaccine. Although mRNA vaccines result in generation of epitopes on the cell membrane that are similar to the ones triggered by the targeted virus [46], the significance of this relation is not yet established. In addition, the mRNA vaccine carries only the code for the spike protein (S), as opposed to the entire SARS-CoV-2 genome [47]. In our study, it is worth noting that all individuals that have been previously diagnosed with COVID-19 by PCR or by serology were excluded. Also, participants that caught SARS-CoV-2 during the study period were excluded, thus minimizing any bias rising from a concomitant COVID-19 infection. Interestingly, a study on SLE patients from Sweden found SARS-CoV-2 antibodies with low neutralizing capacity in pre-pandemic samples [48]. The fact that patients tested positive for anti-SARS-CoV-2 antibodies before the virus spread and thus before the virus was introduced to these patients [48] is intriguing. However, this finding can be explained by two theories. We suggest that this could the result of the autoimmune dysfunction in these patients, whereby autoantibodies are produced against self-antigens and incidentally might have some activity against SARS-CoV-2 antigens. This is referred to as molecular mimicry [18, 19]. We also suggest that these patients might be exposed to other members of the SARS viruses’ family that have spread before SARS-CoV-2. Previous exposure to other coronaviruses that share similar antigens results in the production of antibodies that might bind to a certain extent to the SARS-CoV-2 antigens, thus eliciting an immune response [49]. Coronaviruses are a common cause of upper respiratory tract infections and might have been undetected at the time of infection in these patients [50]. We believe that the very weak neutralizing ability of these antibodies48 supports both theories.
When assessing autoimmune markers in the Lebanese population, the only study conducted at the national level reported that 26.4% of the Lebanese population have an ANA titer ≥ 1:100 in all age groups (ranging from < 18 to > 70 years old) [25]. In the current study, only 8.9% of the participants had an ANA titer ≥ 1:100. The significance of such a difference was not tested as it is not related to the main outcome of this study.
Although the initial trials on the BNT162b2 vaccine confirmed its safety, some adverse events were reported after its approval. The emergence of unexpected side effects could therefore be explained by the lack of prior experience with similar vaccines and limited post-market surveillance. Upon the initiation of the vaccine campaigns, acute anaphylaxis was reported in the UK and the USA, with an incidence of roughly 1 in 100,000 individuals [51]. Shortly thereafter, the CDC recommended that persons with a history of anaphylaxis be excluded from vaccination [52]. Recently, reports of adverse events linked to COVID-19 mRNA vaccines have supported concerns for conditions with a possible autoimmune pathophysiology, including myocarditis [53,54,55], autoimmune hepatitis [56, 57], glomerular disease [58], and panuveitis [59]. However, none of the participants in our study experienced any of the above side effects. In our study, side effects were reported after the first (58.4%) and second dose (46.5%) of the vaccine and included fever, fatigue, myalgias, arthralgias, throat pain, diarrhea, abdominal pain, vomiting, pain at injection site, flu-like symptoms, rash, dizziness, headache, and lymphadenopathy. All these side effects were transient and self-resolving. The initial report by Polack et al. [5] reported similar results. The lack of serious adverse events among this study’s participants can be explained by the relatively small sample size. Most of the reports in the literature are case reports and case series [53, 54, 56,57,58,59] except in the case of myocarditis [55, 60].
These newly detected autoimmune manifestations may be an early alert for the existence of other adverse reactions. Healthcare professionals should therefore be cognizant of the possibility of emerging long-term adverse reactions. Interestingly, most of the reported adverse events of mRNA vaccines [51, 53,54,55,56,57,58,59] can be empirically explained by an autoimmune mechanism. However, the absence of any solid association between vaccines in general and autoimmune dysregulation or autoimmune markers makes the autoimmune theory less likely. Granted, immune dysregulation—which echoes autoimmune diseases’ complex symptoms, presentations, and markers—may be difficult to identify [61].
Elevated autoimmune markers predispose individuals to a higher risk of certain autoimmune diseases [62], although many patients remain seronegative for autoantibodies [63]. This finding can have two explanations; the first explanation is that the autoimmune disease in question, its subtype, or variant is in fact seronegative [63]; the second explanation is that the autoantibodies or markers associated with the autoimmune disease in question are not identified yet [63]. Conversely, individuals with high ANA titers (among other autoimmune markers) do not necessarily suffer from an autoimmune disease [64]. In fact, autoantibodies of different types can be found in normal individuals and sometimes termed natural autoantibodies. These antibodies originate from B1 cells and are thought to be produced as a result of a cross-reaction with bacterial and tumor antigens that are similar to self-antigens. Such antibodies are believed to play a role in sustaining innate immunity and maintaining peripheral tolerance (AMITAL 2007). Thus, it is important to emphasize that autoantibody seropositivity does not necessarily implicate a diagnosis of an autoimmune disorder. Moreover, some asymptomatic individuals with elevated autoimmune markers might be in the initial stages of autoimmune disease and symptoms are expected to flare at any time. They may also be at a phase where the symptoms were attributed to other causes and are currently dormant [65, 66]. Adding to the complexity of the relationship between autoimmune diseases and autoantibodies is the fact that the elevation in certain autoantibodies such as ANA is associated with non-pathologic factors such as female sex and aging [25, 67, 68]. Limitations of the study are mainly related to the sample size as well as the self-reporting of certain variables. However, the inclusion and exclusion criteria implemented reduced the risk of bias from confounding factors including older age, history of immunocompromising condition or medication, and previous COVID-19 infection. Samples from patients were taken before receiving any dose of the BNT162b2 vaccine, after the first dose, and after the second dose. Thus, the effect of the vaccine on ANA and APS titers can be compared to baseline and the effect of added doses can be assessed. Moreover, participants were followed up systematically to survey for any side effect post vaccination. Another strength of the study is related to the selection of participants who were not previously infected with COVID-19, thus providing a clearer picture of the effect of the mRNA vaccine itself on the studied markers.
Conclusion
This study did not find any association between ANA and antiphospholipid antibody levels and the BNT162b2 vaccine. Although the literature does not support an association with autoimmune conditions, larger studies with longer follow-up duration and involving booster doses are required to establish the safety of these vaccines more firmly. Given the massively skewed benefit-to-harm ratio of these vaccines, it is still strongly recommended that all eligible individuals receive their complete COVID-19 vaccination series.
References
Pollard C, De Koker S, Saelens X, Vanham G, Grooten J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol Med. 2013;19(12):705–13.
Meyer M, Huang E, Yuzhakov O, Ramanathan P, Ciaramella G, Bukreyev A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J Infect Dis. 2018;217(3):451–5.
John S, Yuzhakov O, Woods A, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018;36(12):1689–99.
Oliver SE, Gargano JW, Marin M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. Morb Mortal Wkly Rep. 2020;69(50):1922.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Shoenfeld Y, Aharon-Maor A, Sherer Y. Vaccination as an additional player in the mosaic of autoimmunity. Clin Exp Rheumatol. 2000;18(2):181–4.
Tudela P, Martí S, Bonal J. Systemic lupus erythematosus and vaccination against hepatitis B. Nephron. 1992;62(2):236.
Symmons D, Chakravarty K. Can immunisation trigger rheumatoid arthritis? Ann Rheum Dis. 1993;52(12):843.
Ascherio A, Zhang SM, Hernán MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med. 2001;344(5):327–32.
Tourbah A, Gout O, Liblau R, et al. Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology. 1999;53(2):396–401.
Yu O, Bohlke K, Hanson CA, et al. Hepatitis B vaccine and risk of autoimmune thyroid disease: a Vaccine Safety Datalink study. Pharmacoepidemiol Drug Saf. 2007;16(7):736–45.
Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992–1993 and 1993–1994 influenza vaccines. N Engl J Med. 1998;339(25):1797–802.
Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8(3):295–311.
Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2015;3(4):151–5.
Wraith DC, Goldman M, Lambert P-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659–66.
Langridge WH. Edible vaccines. Sci Am. 2000;283(3):66–71.
Todd JA, Wicker LS. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity. 2001;15(3):387–95.
Pascolini S, Vannini A, Deleonardi G, et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Transl Sci. 2021;14(2):502–8.
Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341(27):2068–74.
Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–35.
Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. Exp Med. 2005;202(1):135–43.
Reikine S, Nguyen JB, Modis Y. Pattern recognition and signaling mechanisms of RIG-I and MDA5. Front Immunol. 2014;5:342.
Iacobucci G. Covid-19: Fourth vaccine doses—who needs them and why? BMJ. 2022;376:o30.
EUROIMMUN Medizinische Labordiagnostika AG. ANA diagnostics using indirect immunofluorescence. In: EUROIMMUN, ed. Lubeck (Germany): EUROIMMUN; 2021. https://www.euroimmun.com/documents/Indications/Autoimmunity/Rheumatology/ANA/FA_1510_I_UK_B.pdf. Accessed 10-2-2022.
Racoubian E, Zubaid RM, Shareef MA, Almawi WY. Prevalence of antinuclear antibodies in healthy Lebanese subjects, 2008–2015: a cross-sectional study involving 10,814 subjects. Rheumatol Int. 2016;36(9):1231–6.
ORGENTEC. Anti-phospholipid screen IgG/IgM. ORGENTEC. https://www.orgentec.com/en/products/ELISA/Autoimmune+Disease+Diagnostics/Thrombosis+Diagnostics/ORG+643.html. Published 2022. Accessed 10–2–2022, 2022.
Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73(1):17–23.
Toplak N, Kveder T, Trampuš-Bakija A, Šubelj V, Čučnik S, Avčin T. Autoimmune response following annual influenza vaccination in 92 apparently healthy adults. Autoimmun Rev. 2008;8(2):134–8.
Gatto M, Agmon-Levin N, Soriano A, et al. Human papillomavirus vaccine and systemic lupus erythematosus. Clin Rheumatol. 2013;32(9):1301–7.
Palmieri B, Poddighe D, Vadala M, Laurino C, Carnovale C, Clementi E. Severe somatoform and dysautonomic syndromes after HPV vaccination: case series and review of literature. Immunol Res. 2017;65(1):106–16.
Khamaisi M, Shoenfeld Y, Orbach H. Guillain-Barre syndrome following hepatitis B vaccination. Clin Exp Rheumatol. 2004;22(6):767–70.
Genovese C, La Fauci V, Squeri A, Trimarchi G, Squeri R. HPV vaccine and autoimmune diseases: systematic review and meta-analysis of the literature. J Prev Med Public Health. 2018;59(3):E194.
Liu EY, Smith LM, Ellis AK, et al. Quadrivalent human papillomavirus vaccination in girls and the risk of autoimmune disorders: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2018;190(21):E648–55.
Miranda S, Chaignot C, Collin C, Dray-Spira R, Weill A, Zureik M. Human papillomavirus vaccination and risk of autoimmune diseases: a large cohort study of over 2 million young girls in France. Vaccine. 2017;35(36):4761–8.
Soldevilla H, Briones S, Navarra S. Systemic lupus erythematosus following HPV immunization or infection? Lupus. 2012;21(2):158–61.
Poddighe D, Castelli L, Marseglia GL, Bruni P. A sudden onset of a pseudo-neurological syndrome after HPV-16/18 AS04-adjuvated vaccine: might it be an autoimmune/inflammatory syndrome induced by adjuvants (ASIA) presenting as a somatoform disorder? Immunol Res. 2014;60(2):236–46.
Bobba RS, Johnson SR, Davis AM. A review of the Sapporo and revised Sapporo criteria for the classification of antiphospholipid syndrome Where do the revised Sapporo criteria add value? J Rheumatol. 2007;34(7):1522–7.
Blank R HR, Castillo R, Samanovic m, Vasudevanpillai Girija P, Rackoff P, Solomon G, Azar N, Rosenthal P, Izmirly P, Samuels J, Golden B, Reddy S, Abramson S, Mulligan M, Scher J. Low incidence and transient elevation of autoantibodies post mRNA COVID-19 vaccination [abstract]. Abstract presented at American College of Rheumatology, Empowering Rheumatology Professionals; Tuesday, November 9, 2021, 2021.
Sacker A, Kung V, Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100(2):471–2.
Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100(2):473–4.
Gupta RK, Ellis BK. Concurrent antiglomerular basement membrane nephritis and antineutrophil cytoplasmic autoantibody–mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int Rep. 2022;7(1):127–8.
Thurm C, Reinhold A, Borucki K, et al. Homologous and heterologous anti-COVID-19 vaccination does not induce new-onset formation of autoantibodies typically accompanying lupus erythematodes, rheumatoid arthritis, celiac disease and antiphospholipid syndrome. Vaccines. 2022;10(2):333.
Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5(11):648–52.
Edwards DK, Jasny E, Yoon H, et al. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J Transl Med. 2017;15(1):1–18.
Pepini T, Pulichino A-M, Carsillo T, et al. Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J Immunol. 2017;198(10):4012–24.
Marć MA, Domínguez-Álvarez E, Gamazo C. Nucleic acid vaccination strategies against infectious diseases. Expert Opin Drug Deliv. 2015;12(12):1851–65.
Xia X. Domains and functions of spike protein in SARS-Cov-2 in the context of vaccine design. Viruses. 2021;13(1):109.
Sjöwall J, Azharuddin M, Frodlund M, et al. SARS-CoV-2 antibody isotypes in systemic lupus erythematosus patients prior to vaccination: associations with disease activity, antinuclear antibodies, and immunomodulatory drugs during the first year of the pandemic. Front Immunol. 2021:3373.
Huang J, Teoh JY-C, Wong SH, Wong M. The potential impact of previous exposure to SARS or MERS on control of the COVID-19 pandemic. Eur J Epidemiol. 2020;35(11):1099–103.
Greenberg SB. Update on human rhinovirus and coronavirus infections. Semin Respir Crit Care. 2016;37(4):555–71.
Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–9.
Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020. Centers for Disease Control and Prevention (CDC). Morbidity and Mortality Weekly Report (MMWR) Web site. https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm#suggestedcitation. Published 2021. Updated 6–1–2021. Accessed 13–2–2022, 2022.
Koizumi T, Awaya T, Yoshioka K, et al. Myocarditis after COVID-19 mRNA vaccines. QJM. 2021;114(10):741–3.
Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–6.
Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385(23):2140–9.
Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021;123:102706.
Goulas A, Kafiri G, Kranidioti H, Manolakopoulos S. A typical autoimmune hepatitis (AIH) case following COVID-19 mRNA vaccination More than a coincidence? Liver Int. 2022;42(1):254–5.
Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. 2021;100(5):959–65.
Mudie LI, Zick JD, Dacey MS, Palestine AG. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;29(4):741–2.
Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–40.
Noureldine H, Nour-Eldine W, Hodroj M, Noureldine M, Taher A, Uthman I. Hematological malignancies in connective tissue diseases. Lupus. 2020;29(3):225–35.
Scofield RH. Autoantibodies as predictors of disease. Lancet. 2004;363(9420):1544–6.
Alessandri C, Conti F, Conigliaro P, Mancini R, Massaro L, Valesini G. Seronegative autoimmune diseases. Ann N Y Acad Sci. 2009;1173(1):52–9.
Tan E, Feltkamp T, Smolen J, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40(9):1601–11.
Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33.
Edwards C, Syddall H, Jameson K, et al. The presence of anticardiolipin antibodies in adults may be influenced by infections in infancy. QJM. 2008;101(1):41–7.
Li Q-Z, Karp DR, Quan J, et al. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13(2):1–11.
Guo Y-P, Wang C-G, Liu X, et al. The prevalence of antinuclear antibodies in the general population of china: a cross-sectional study. Curr Ther Res. 2014;76:116–9.
Funding
Laboratory testing kits were provided by “Droguerie de l’Union SAL” © (Hadath, Lebanon).
Author information
Authors and Affiliations
Contributions
HAN: study design, patient recruitment, data collection, data analysis, manuscript writing.
JM: study conceptualization, study design, data collection, manuscript writing.
MEH: data collection, data analysis, manuscript writing.
GC: data collection, patient recruitment, data analysis, manuscript writing.
JEM: study conceptualization, study design, study oversight, manuscript review.
All authors reviewed, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noureldine, H.A., Maamari, J., El Helou, M.O. et al. The effect of the BNT162b2 vaccine on antinuclear antibody and antiphospholipid antibody levels. Immunol Res 70, 800–810 (2022). https://doi.org/10.1007/s12026-022-09309-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09309-5