Summary
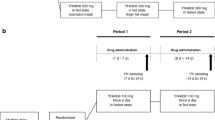
The effect of food on the pharmacokinetic profile of the nonsteroidal anti-inflammatory drug meloxicam was investigated in 2 open, randomised, crossover studies conducted in 2 different groups of healthy male volunteers. A pilot study assessed the effect of a standardised continental breakfast on the pharmacokinetics of a single oral dose of meloxicam 30mg in 6 volunteers, while the other study investigated the pharmacokinetics of a single oral dose of meloxicam l5mg after a high-fat breakfast in 16 volunteers. The extent of meloxicam absorption was not affected by either the continental or the high-fat breakfast. Maximum plasma drug concentrations tended to be achieved earlier after food, and the onset of absorption was slightly delayed after the high-fat meal. However, none of these changes was considered clinically relevant and bioequivalence was demonstrated for both situations (fasted and after high-fat meal). Disposition parameters such as mean residence time, elimination half-life, total clearance or volume of distribution during the terminal phase did not change. Both doses of meloxicam were well tolerated in the fed and the fasting states. In conclusion, the single dose pharmacokinetics of oral meloxicam were not significantly affected by food, suggesting that the drug may be given with or after meals.
Similar content being viewed by others
References
Engelhardt G, Schlegel K, Schnitzler Chr. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent. Inflamm Res. In press
Türck D, Busch U, Heinzel G, et al. Basic clinical pharmacokinetics of meloxicam, a new NSAID. Scand J Rheumatol 1994; 98Suppl.: 120
Welling P. Influence of food and diet on gastrointestinal drug absorption: A review. J Pharmacokin Biopharm 1977; 5: 219–334
Wistanley PA, Orme MLE. The effect of food on drug bioavailability. Br J Clin Pharmacol 1989; 28: 621–8
FDA, Division of Bioequivalence. Guidance: Oral extended (controlled) release dosage forms: in vivo bioequivalence and in vitro dissolution testing. Rockville: 1993
Edgar B. Design of food studies. In: Midha K, Blume H, editors. Bio-international. Bioavailability, bioequivalence and pharmacokinetics. Stuttgart: Medpharm Scientific Publishers, 1993
Palma R, Vidon N, Houin G, et al. Influence of bile salts and lipids on intestinal absorption of griseofulvin in man. Eur J Clin Pharmacol 1986; 31: 319–25
Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. 4th ed. London: Lea and Febiger, 1991
Steinijans VW, Sauter R. Food studies: acceptance criteria and statistics. In: Midha K, Blume H, editors. Bio-international. bioavailability, bioequivalence and pharmacokinetics. Stuttgart: Medpharm Scientific Publishers, 1993
Schulz HU, Karlsson S, Sahner-Ahrens I, et al. Effect of drug intake prior to or after meals on serum theophylline concentrations: Single dose studies with Euphyllong. Int J Clin Pharmacol Ther Toxicol 1987; 25: 222–8
Blume H, Siewert M, Steinijans VW, et al. Bioäquivalenz von per os applizierten Retard-Arznreimitteln: Konzeption der Studien und Entscheidung über Austauschbarkeit. Pharm Ind 1989; 51: 1025–33
Schuirman DJ. A Comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. Pharmacokinet Biopharmaceut 1987; 15: 657–80
Hauschke D, Steinijans VW, Diletti E. A distribution-free procedure for the statistical analysis of bioequivalence studies. Int J Clin Pharmacol Ther Toxicol 1990; 28: 72–8
Steinijans VW, Trautmann H, Johnson E, et al. Theophylline steady state pharmacokinetics: Recent concepts and their application in chronotherapy of reactive airway diseases. Chronobiol Int 1987: 4: 331–47
Velicitat P, Auvinet B, Ziller R, et al. A comparison of the onset and intensity of action of meloxicam ampoules and meloxicam capsules. Br J Rheumatol. Submitted for publication
Yakatan G. Pharmacology and pharmacokinetics of isoxicam. Semin Arthritis Rheum 1982; 12Suppl. 2: 1544–59
Ishizaki T, Nomura T, Abe T. Pharmacokinetics of piroxicam, a new nonsteroidal anti-inflammatory agent, under fasting and postprandial states in man. J Pharmacokinet Biopharm 1979; 4: 369–81
Francis RJ, Dixon JS, Lowe JR, et al. The effect of food and of antacid on the single oral dose pharmacokinetics of tenoxicam. Eur J Drug Metab Pharmacokinet 1985; 10: 309–14
Francis RJ, Allen JG, Looi D, et al. Pharmacokinetics of tenoxicam after oral administration in healthy human subjects of various single doses. Eur J Drug Metab Pharmacokinet 1987; 12: 59–63
Acknowledgements
The authors would like to thank Mrs C. Huber for her excellent performance of the analytical procedures.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Türck, D., Busch, U., Heinzel, G. et al. Effect of Food on the Pharmacokinetics of Meloxicam after Oral Administration. Clinical Drug Investigation 9, 270–276 (1995). https://doi.org/10.2165/00044011-199509050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199509050-00004