Abstract
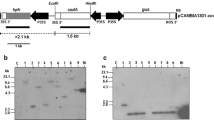
A search of compounds capable of inducing specific gene expression in plants without affecting growth and development led to the examination of changes in the pattern of gene expression in corn after treatment with substituted benzenesulfonamide herbicide safeners. Following hydroponic treatment of corn with the safener N-(aminocarbonyl)-2-chlorobenzenesulfonamide (2-CBSU), the specific induction of new translatable mRNA species was observed. Replicate copies of a cDNA library made using RNA from 2-CBSU-treated corn roots were differentially screened with cDNA probes made from either the same mRNA fraction used for library construction or mRNA isolated from roots treated with 2-chlorobenzenesulfonamide (2-CBSA), an inactive analog of the safener. Colonies showing hybridization only with the probe made using mRNA from 2-CBSU-treated roots were further characterized to assess the specificity of the induction and decay of the corresponding induced RNA species. RNA blot analyses showed two clones, designated In2-1 and In2-2, contained plasmids that hybridized to RNAs that were induced from an undetectable background in corn roots within 30 minutes after treatment with 2-CBSU. Leaf and meristem tissues showed similar inductions of the In2-1 and In2-2 RNA species after a delay of several hours. In addition, both RNA species were induced in corn by foliar application of 2-CBSU. In contrast, neither RNA species was induced following stress treatments of plants. These results indicate a substituted benzenesulfonamide safener might be used with the promoters from the In2-1 and In2-2 genes to develop a new inducible gene expression system for plants.
Similar content being viewed by others
References
Amuti K, Sweetser PB: Herbicidal Antidotes. United States Patent US 4,645,527 (1987).
Barker, RF, Idler, KB, Thompson, DV, Kemp, JD: Nucleotide sequence of the T-DNA region from the Agrobacterium tumefaciens octopine Ti plasmid PTi15955. Plant Mol Biol 2: 335–350 (1983).
Baumann, G, Raschke, E, Bevan, M, Schöffl, F: Functional analysis of sequences required for transcriptional activation of a soybean heat shock gene in transgenic tobacco. EMBO J 6: 1161–1166 (1987).
Bol JF, Cornelissen BJ, van Kan, JA: Recombinant DNA; Transformed microorganisms, plant cells and plants; a process for introducing an inducible property in plants, and a process for producing a polypeptide or protein by means of plants or plant cells. European Patent Application Publication Number EP 0337532 A1 (1990).
Brinster, RL, Chen, HY, Warren, R, Sarthy, A, Palmiter, RD: Regulation of metallothionein-thymidine kinase fusion plasmids injected into mouse eggs. Nature 296: 39–42 (1982).
Broglie, KE, Biddle, P, Cressman, R, Broglie, R: Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell 1: 599–607 (1989).
Colbert, JT, Hershey, HP, Quail, PH: Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci USA 45: 1703–1708 (1983).
Colbert, JT, Hershey, HP, Quail, PH: Phytochrome regulation of phytochrome mRNA abundance. Plant Mol Biol 5: 91–101 (1985).
Ellis, JF, Peek, JW, Boehle, J, Muller, G: Effectiveness of a new safener for protecting sorghum (Sorghum bicolor) from metolachlor injury. Weed Sci 28: 1–5 (1980).
Ellis, JG, Llewellyn, DJ, Dennis, ES, Peacock, WJ: Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J 6: 11–16 (1987).
Fluhr, R, Kuhlemeier, CG, Nagy, T, Chua, N-H: Organ-specific and light-induced expression of plant genes. Science 232: 1106–1112 (1986).
Hagan, G, Guilfoyle, TJ: Rapid induction of selective transcription by auxins. Mol Cell Biol 5: 1197–1203 (1985).
Hatzlos, KK: Herbicide antidotes: development, chemistry, and mode of action. Adv Agron 36: 265–316 (1983).
Herrora-Estrella, L, Van den, Broeck, G, Maenhaut, R, Van, Montagu, M, Schell, J, Timko, M, Cashmore, A: Light-inducible and chloroplast-associated expression of a chimaeric gene introduced into Nicotiana tabacum using a Ti plasmid vector. Nature 310: 115–120 (1984).
Hershey, HP, Colbert, JT, Lissemore, JL, Barker, RF, Quail, PH: Molecular cloning of cDNA for Avena phytochrome. Proc Natl Acad Sci USA 81: 2332–2336 (1984).
Hershey, HP, Quail, PH: Identification of cDNA clones representing phytochrome and other low abundance red-light regulated sequences. In: Weissbach, A, Weissbach, H (eds) Methods in Enzymology, vol 118, pp. 369–383. Academic Press, Orlando, FL (1986).
Howard, EA, Walker, JC, Dennis, ES, Peacock, WJ: Regulated expression of an alcohol dehydrogenase 1 chimeric gene introduced into maize protoplasts. Planta 170: 535–540 (1987).
Lay, MM, Cassida, JL: Dichloroacetamide antidotes enhance thiocarbamate sulfoxide detoxification by elevating corn root glutathione content and glutathione S-transferase activity. Pest Biochem Physiol 6: 442–436 (1976).
Marcotte, WR, Bayley, CC, Quatrano, RS: Regulation of a wheat promoter by abscisic acid in rice protoplasts. Nature 335: 454–457 (1988).
Maxam, AM, Gilbert, W: Sequencing end-labeled DNA with base-specific chemical cleavages. In: Grosman, L, Moldave, K (eds) Method in Enzymology, vol 65, pp. 499–560. Academic Press, New York (1980).
Murray, MG, Peters, DL, Thompson, WF: Ancient repeated sequences in the pea and mung bean genomes and implications for genome evolution. J Mol Evol 17: 31–42 (1981).
Parker, C: Herbicide antidotes — a review. Pestic Sci 14: 40–48 (1983).
Pelham, HRB: A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell 30: 517–528 (1982).
Ryals J, Harms C, Duesing J, Sperisen C, Meins F, Payne G: Chemically regulatable DNA sequences and genes and uses thereof. European Patent Application EP 0332104 A2 (1990).
Sanger, F, Milkin, S, Coulson, AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Severin, K, Schöffl, F: Heat-inducible hygromycin resistance in transgenic tobacco. Plant Mol Biol 15: 827–833 (1990).
Sweetser, PB: Safening of sulfonylurea herbicides to cereal crops: Mode of herbicide antidote action. Weeds 3: 1147–1154 (1985).
Sweetser, PB, Schow, GS, Hutchison, JM: Metabolism of chlorsulfuron by plants: biological basis for selectivity of a new herbicide for cereals. Pest Biochem Physiol 17: 18–23 (1982).
Taylor, JL, Fritzemeier, K-H, Haeuser, I, Kombrink, E, Rohwer, F, Schroeder, M, Strittmatter, G, Hahlbrock, K: Structural analysis and activation by fungal infection of a gene encoding a pathogenesis-related protein in potato. Mol Plant-Microbe Interact 3: 72–77 (1990).
Vavrina, CS, Phatak, SC: Efficacy of triapenthenol as a safener against metribuzin injury in soybeam (Glycine max) cultivars. J Plant Growth Regul 7: 67–75 (1988).
Weigland, RC, Shah, DM, Mozer, TJ, Harding, EI, Diaz-Collier, J, Saunders, C, Jaworski, EG, Tiemeier, DC: Messenger RNA encoding a glutathione S-transferase responsible for herbicide tolerance in maize is induced in response to safener treatment. Plant Mol Biol 7: 235–243 (1986).
Young, RA, Davis, RW: Yeast RNA polymerase II genes: isolation with antibody probes. Science 222: 778–781 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hershey, H.P., Stoner, T.D. Isolation and characterization of cDNA clones for RNA species induced by substituted benzenesulfonamides in corn. Plant Mol Biol 17, 679–690 (1991). https://doi.org/10.1007/BF00037053
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037053