Abstract
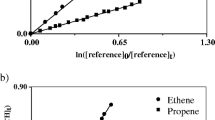
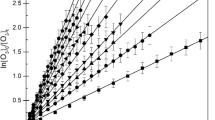
The design and performance of a smog chamber for the study of photochemical reactions under simulated environmental conditions is described. The chamber is thermostated for aerosol experiments, and it comprises a gas chromatographic sample enrichment system suitable for monitoring hydrocarbons at the ppbv level. By irradiating NO x /alkane-mixtures rate constants for the reaction of OH radicals with n-alkanes are determined from n-pentane to n-hexadecane to be (k±2σ)/10−12 cm3 s−1=4.29±0.16, 6.2±0.6, 7.52 (reference value), 8.8±0.3, 10.2±0.3, 11.7±0.4, 13.7±0.3, 15.1±0.5, 17.5±0.6, 19.3±0.7, 22.3±1.0, and 25.0±1.3, respectively at 312 K. Rate constants, (k±2σ)/10−17 cm3 s−1, for the reaction of ozone with trans-2-butene (21.2±1.0), cis-3-methylpentene-(2) (47.2±1.7), cyclopentene (62.4±3.5), cyclohexene (7.8±0.5), cycloheptene (28.3±1.5), α-pinene (8.6±1.3), and β-pinene (1.4±0.2) are determined in the dark at 297 K using cis-2-butene (13.0) as reference standard.
Similar content being viewed by others
References
Adeniji, S. A., Kerr, J. A., and Williams, M. R., 1981, Rate constants for ozone-alkene reactions under atmospheric conditions, Int. J. Chem. Kinet. 13, 209–217.
Atkinson, R., 1986a, Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions, Chem. Rev. 86, 69–201.
Atkinson, R., 1986b, Estimations of OH radical rate constants from H-atom abstraction from C-H and O-H-bonds over the temperature range 250–1000 K, Int. J. Chem. Kinet. 18, 555–568.
Atkinson, R., Aschmann, S. M., and Carter, W. P. L., 1983a, Effects of ring strain on gas-phase rate constants. 2. OH radical reactions with cycloalkenes, Int. J. Chem. Kinet. 15, 1161–1177.
Atkinson, R., Aschmann, S. M., Carter, W. P. L., and PittsJr., J. N., 1983b, Effects of ring strain on gas-phase rate constants. I. Ozone reactions with cycloalkenes, Int. J. Chem. Kinet. 15, 721–731.
Atkinson, R., Aschmann, S. M., Carter, W. P. L., Winer, A. M., and Pitts, J. N.Jr., 1982a, Kinetics of the reactions of OH radicals with n-alkanes at 299±2 K, Int. J. Chem. Kinet. 14, 781–788.
Atkinson, R., Aschmann, S. M., Winer, A. M., and PittsJr., J. N., 1984, Gas phase reactions of NO2 with alkenes and dialkenes, Int. J. Chem. Kinet. 16, 697–706.
Atkinson, R., Aschmann, S. M., Winer, A. M., and PittsJr., J. N., 1985, Kinetics and atmospheric implications of the gas-phase reactions of NO3 radicals with a series of monoterpenes and related organics at 294±2 K, Environ. Sci. Technol. 19, 159–163.
Atkinson, R. and Carter, W. P. L., 1984, Kinetics and mechanisms of gas-phase reactions of ozone with organic compounds under atmospheric conditions, Chem. Rev. 84, 437–470.
Atkinson, R., Winer, A. M., and PittsJr., J. N., 1982b, Rate constants for the gas phase reactions of O3 with the natural hydrocarbons isoprene and α- and β-pinene, Atmos. Environ. 16, 1017–1020.
Behnke, W., Holländer, W., Koch, W., Nolting, F., and Zetzsch, C., 1986a, A smog chamber for studies of the photochemical degradation of chemicals in the presence of aerosol surfaces, (submitted).
Behnke, W., Nolting, F., and Zetzsch, C., 1986b, unpublished results.
Cadle, R. D. and Schadt, C., 1952, Kinetics of the gas phase reaction of olefines with ozone, J. Am. Chem. Soc. 74, 6002–6004.
Dimitriades, B., 1981, The role of natural organics in photo-chemical air pollution. Issues and research needs, J. Air Pollut. Control. Ass. 31, 229–235.
Gaffney, J. S., Atkinson, R., and PittsJr., J. N., 1975, Relative rate constants for the reaction of O(3P) atoms with selected olefins, monoterpenes, and unsaturated aldehydes. J. Am. Chem. Soc. 97, 5049–5051.
Graedel, T. E., 1979, Terpenoids in the atmosphere, Rev. Geophys. Space Phys. 17, 937–947.
Grimsrud, E. P., Westberg, H. H., and Rasmussen, R. A., 1975, Atmospheric reactivity of monoterpene hydrocarbons, NO x photooxidation and ozonolysis, Int. J. Chem. Kinet. Symp. 1, 183–195.
Holländer, W., Behnke, W., Koch, W., and Pohlmann, G., 1984, in Proc. Third European Sympos, on Physico-Chemical Behaviour of Atmospheric Pollutants, B., Versino and G., Angeletti (eds.), D. Reidel, Dordrecht, pp. 309–319.
Japar, S. M., Wu, C. H., and Niki, H., 1974a, Rate constants for the reaction of ozone with olefins in the gas phase, J. Phys. Chem. 78, 2318–2320.
Japar, S. M., Wu, C. H., and Niki, H., 1974b, Rate constants for the gas phase reaction of ozone with α-pinene and terpinolene, Environ. Lett. 7, 245–249.
Kleindienst, T. E., Harris, G. W., and PittsJr., J. N., 1982, Rates and temperature dependences of the reaction of OH with isoprene, its oxidation products, and selected terpenes, Environ. Sci. Technol. 16, 844–846.
Lurmann, F. W., Nitta, B., Ganesan, K., and Lloyd, A. C., 1984, Modelling potential ozone impacts from natural sources — III. Ozone modelling in Tampa/St. Petersburg, Florida, Atmos. Environ. 18, 1133–1143.
Rasmussen, R. A., 1972, What do the hydrocarbons from trees contribute to air pollution?, J. Air Poll. Control Assoc. 22, 537–543.
Ravishankara, A. R., Wagner, S., Fischer, S., Smith, G., Schiff, R., Watson, R. T., Tesi, G., and Davis, D. D., 1978, A kinetic study of the reactions of OH with several aromatic and olefinic compounds, Int. J. Chem. Kinet. 10, 783–804.
Ripperton, L. A., Jeffries, H. E., and White, O., 1972, Formation of aerosols by reaction of ozone with selected hydrocarbons, Adv. Chem. Ser., No. 113, 219–231.
Weast, R. C., Astle, M. J., and Beyer, W. H., 1983, CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, Florida, p. C324.
Whitby, R. A. and Coffey, P. E., 1977, Measurements of terpenes and other organics in an Adirondack Mountain pine forest, J. Geophys. Res. 82, 5928–5934.
Winer, A. M., Lloyd, A. C., Darnall, K. R., and PittsJr., J. N., 1976, Relative rate constants for the reaction of the hydroxyl radical with selected ketones, chloroethenes, and monoterpene hydrocarbons, J. Phys. Chem. 80, 1635–1639.
Zimmerman, P. R., Chatfield, R. B., Fishman, J., Crutzen, P. J., and Hanst, P. L., 1978, Estimates of the production of CO and H2 from the oxidation of hydrocarbon emissions from vegetation, Geophys. Res. Lett. 5, 679–682.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nolting, F., Behnke, W. & Zetzsch, C. A smog chamber for studies of the reactions of terpenes and alkanes with ozone and OH. J Atmos Chem 6, 47–59 (1988). https://doi.org/10.1007/BF00048331
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00048331