Summary
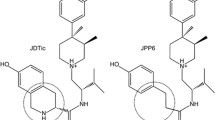
Based upon the message-address concept, this molecular modeling study used the δ-selective agonist spiroindanyloxymorphone (SIOM) as a molecular template for a conformational search and analysis of δ-selective opioid peptides. It was assumed that the tyramine moiety plays the same role for δ-opioid receptor recognition in both peptide and non-peptide ligands. Using 20 reported low-energy conformations of Tyr-cyclo[d-Cys-d-Pen]-OH (JOM-13) for comparison, the geometrical relationship of the two aromatic rings present in SIOM was used for the identification of potential active conformations of JOM-13, from which two δ-receptor-binding models (I and II) were constructed. Models I and II differ from each other in the arrangement of the peptide backbones. To evaluate the two models, a conformational search of two other known δ-selective ligands, [d-Pen2,d-Pen5]enkephalin (DPDPE) and [d-Pen2,l-Pen5]enkephalin (DPLPE) was performed, using the geometrical relationship of the two aromatic rings defined in the two receptor-binding models as a molecular template. Among the conformations generated from the molecular simulation, low-energy conformers of DPDPE and DPLPE conforming to models I and II were identified. Unlike model I, conformers of DPDPE and DPLPE that fit model II contain a cis amide bond in the Gly3 residue.
Similar content being viewed by others
References
SchwyzerR., Ann. New York Acad. Sci., 247 (1977) 3.
HrubyV.J. and GehrigC.A., Med. Res. Rev., 9 (1989) 343.
SchillerP.W., In HerzA. (Ed.) Opioids I, Vol. 104, Springer, Berlin, Germany, 1993, pp. 681–710.
SalvadoriS., BryantS.D., BianchiC., BalboniG., ScaranariV., AttilaM. and LazarusL.H., J. Med. Chem., 36 (1993) 3748.
QianX., KövérK.E., ShenderovichM.D., LouB., MisickaA., ZalewskaT., HorváthR., DavisP., BilskyE.J., PorrecaF., YamamuraH.I. and HrubyV.J., J. Med. Chem., 37 (1994) 1746.
BryantS.D., AttilaM., SalvadoriS., GuerriniR. and LazarusL.H., Pept. Res., 7 (1994) 175.
MisickaA., LipkowskiA.W., HorvathR., DavisP., YamamuraH.I., PorrecaF. and HrubyV.J., J. Med. Chem., 37 (1994) 141.
LordJ.A.H., WaterfieldA.A., HughesJ. and KosterlitzH.W., Nature, 267 (1977) 495.
ChuangL.C., ChenS.T. and YaC., Biochim. Biophys. Acta, 1158 (1993) 209.
SchillerP.W. and DiMaioJ., Nature, 297 (1982) 74.
BradburyA.F., SmythD.G. and SnellC.R., Nature, 260 (1976) 165.
GorinF.A. and MarshallG.R., Proc. Natl. Acad. Sci. USA, 74 (1977) 5179.
SchillerP.W., YamC.F. and LisM., Biochemistry, 16 (1977) 1832.
GottschlichR., Kontakte, 1 (1990) 3.
HrubyV.J., Biopolymers, 33 (1993) 1073.
SchillerP.W., NguyenT.M., WeltrowskaG., WilkesB.C., MarsdenB.J., LemieuxC. and ChungN.N., Proc. Natl. Acad. Sci. USA, 89 (1992) 11871.
MosbergH.I. and KroonaH.B., J. Med. Chem., 35 (1992) 4498.
MosbergH.I., LomizeA.L., WangC., KroonaH., HeylD.L., Sobczy-KijiroK., MaW., MousigianC. and PorrecaF., J. Med. Chem., 37 (1994) 4371.
MosbergH.I., OmnassJ.R., LomizeA.L., HeylD.L., NordanI., MousigianC., DavisP. and PorrecaF., J. Med. Chem., 37 (1994) 4384.
MosbergH.I., HurstR., HrubyV.I., GeeK., YamamuraH.I. and GalliganJ.J., Proc. Natl. Acad. Sci. USA, 80 (1983) 5871.
HrubyV.J., KaoL., PettittB.M. and KarplusM., J. Am. Chem. Soc., 110 (1988) 3351.
MatsunagaT.O., CollinsN., RamaswamiV., YamamuraS.H., O'brienD.F. and HrubyV.J., Biochemistry, 32 (1993) 13180.
Flippen-AndersonJ.L., HrubyV.J., CollinsN., GeorgeC. and CudneyB., J. Am. Chem. Soc., 116 (1994) 7523.
PettittB.M., MatsunagaT., Al-ObeidiF., GehrigC., HrubyV.J. and KarplusM., Biophys. J., 60 (1991) 1540.
FroimowitzM., Biopolymers, 30 (1990) 1011.
ChewC., VillarH. and LoewG., Mol. Pharmacol., 39 (1991) 502.
SmithP.E. and PettittB.M., J. Am. Chem. Soc., 113 (1991) 6029.
WilkesB.C. and SchillerP.W., J. Comput.-Aided Mol. Design, 5 (1991) 293.
MosbergH.I., Sobczy-KijiroK., SubramanianP., CrippenG.M., RamalingamK. and WoodardR.W., J. Am. Chem. Soc., 112 (1990) 822.
SmithP.E. and PettittB.M., Biopolymers, 32 (1992) 1623.
NikiforovichG.V., HrubyV.J., PrakashO. and GehrigC.A., Biopolymers, 31 (1991) 941.
NikiforovichG.V., GolbraikhA.A., ShenderovichM.D. and BalodisJ., Int. J. Pept. Protein Res., 36 (1990) 209.
LomizeA.L., Flippen-AndersonJ.L., GeorgeC. and MosbergH.I., J. Am. Chem. Soc., 116 (1994) 429.
PolinskyA., CooneyM.G., Toy-PalmerA., ÖsapayG. and GoodmanM., J. Med. Chem., 35 (1992) 4185.
StewartD.E., SarkarA. and WamplerJ., J. Mol. Biol., 214 (1990) 253.
ElielE.L., WilenS.H., ManderL.N., In Stereochemistry of Organic Compounds, Wiley, New York, NY, 1994, pp. 620–621.
KawaiM., PottorfR.S. and RichD.H., J. Med. Chem., 29 (1986) 2409.
MierkeD.F., YamazakiT., Said-NejadO.E., FelderE.R. and GoodmanM., J. Am. Chem. Soc., 111 (1989) 6847.
YamazakiT., RoS., GoodmanM., ChuangN.N. and SchillerP.W., J. Med. Chem., 36 (1993) 708.
CerriniS., GavuzzoE., LucenteG. and PinnenF., Int. J. Pept. Protein Res., 31 (1988) 447.
SpencerR.G., HalversonK.J., AugerM., MaDermottA.E., GriffinR.G. and LansburyP.T.J., Biochemistry, 30 (1991) 10382.
MosbergH.I., OmnaasJ.R., SmithC.B. and MedzihradskyF., Life Sci., 43 (1988) 1013.
PortogheseP.S., SultanaM. and TakemoriA.E., J. Med. Chem., 33 (1990) 1714.
PortogheseP.S., MoeS.T. and TakemoriA.E., J. Med. Chem., 36 (1993) 2572.
PortogheseP.S., NagaseK.E., MaloneyHussK.E., LinC. and TakemoriA.E., J. Med. Chem., 34 (1991) 1715.
PortogheseP.S., J. Med. Chem., 35 (1992) 1927.
PortogheseP.S., SultanaM., MoeS.T. and TakemoriA.E., J. Med. Chem., 37 (1994) 579.
StewartP.E. and HammondD.L., J. Pharmacol. Exp. Ther., 266 (1993) 820.
KongH.Y., RaynorK., YasudaK., MoeS.T., PortogheseP.S., BellG.I. and ReisineT., J. Biol. Chem., 268 (1993) 23055.
ChangK.J., RigdonG.C., HowardJ.L. and McNuttR.W., J. Pharmacol. Exp. Ther., 267 (1993) 852.
KenakinT., Trends Pharmacol. Sci., 16 (1995) 188.
KenakinT., Trends Pharmacol. Sci., 16 (1995) 232.
LeffP., Trends Pharmacol. Sci., 16 (1995) 89.
Stewart, J.J.P., MOPAC MANUAL, Frank J. Seiler Research Laboratory, United States Air Force Academy, CO.
Dauber-OsguthorpeP., RobertsV.A., OsguthorpeP.J., WolffJ., GenestM. and HaglerA.T., Proteins Struct. Funct. Genet., 4 (1988) 31.
FletchR., Practical Methods of Optimization, Vol. 1, Wiley, New York, NY, 1980.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gao, P. Comparison of cyclic δ-opioid peptides with non-peptide δ-agonist spiroindanyloxymorphone (SIOM) using the message-address concept: A molecular modeling study. J Computer-Aided Mol Des 10, 327–336 (1996). https://doi.org/10.1007/BF00124502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00124502