Abstract
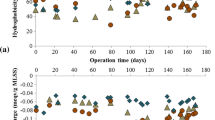
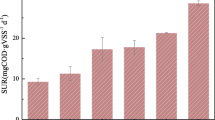
The enzymatic activity of activated sludge was investigated with special emphasis on the localization of the enzymes in the sludge floc matrix. Activated sludge from an advanced activated-sludge treatment plant, performing biological N and P removal, was used. An enzymatic fingerprint was established using a panel of six different enzymes. The fingerprint revealed peptidase as the most dominating specific enzyme tested. By monitoring sludge bulk enzymatic activity over a 3-month period using fluorescein diacetate as an enzyme substrate, considerable variations in activity were observed even over short periods (a few days). The variation in esterase activity was to some extent correlated to the presence of humic compounds in the sludge, but not to the sludge protein content. Comparison of full sludge enzyme activity to the activity of a batch-grown sludge culture indicated that enzymes accumulated in sludge flocs. A large proportion of the exoenzymes were immobilized in the sludge by adsorption in the extracellular polymeric substances (EPS) matrix. This was demonstrated by extraction of EPS from the activated sludge using cation exchange. Contemporary to the release of EPS a very large fraction of the exoenzymes was released into the water. This showed that the exoenzymes should be considered to be an integrated part of the EPS matrix rather than as direct indicators of the microbial activity or biomass.
Similar content being viewed by others
References
APHA, AWWA, and WEF (1992) Standard methods for examination of water and wastewater, 18th edn. American Public Health Association, Baltimore, Md
Boczar BA, Begley WM and Larson RJ (1992) Characterization of enzyme activity in activated sludge using rapid analyses for specific hydrolases. Water Environ Res 64:792–797
Box JD (1983) Investigation on the Folin-Ciocalteau phenol reagent for determination of polyphenolic substances in natural waters. Water Res 17:511–525
Bruus JH, Nielsen PH, Keiding K (1992) On the stability of activated sludge flocs with implication to dewatering. Water Res 26:1597–1604
Chróst RJ (1991) Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chróst RJ (ed) Microbial enzymes in aquatic environments. Springer, New York Berlin Heidelberg p. 29
Dreywood R (1946) Qualitative test for carbohydrate material. Ind Eng. Chem 18:499
Frølund B, Keiding K (1994). Implementation of an HPLC polysterene divinylbenzene column for separation of activated sludge exopolymers. Appl Microbiol Biotechnol 41:708–716
Goodwin JAS, Forster CF (1985) A further examination into the composition of activated sludge surfaces in relation to their settlement characteristics. Water Res 19:527–533
Harrëmoes P, Bundgaard E, Hence M (1991) Developments in wastewater treatment for nutrient removal. J European Water Pollut Control Fed 1:19–23
Hobbie JE, Daley RJ, Jasper S (1977) Use of nuclepore filters for counting bacteria by flourescens microscopy. Appl Environ Microbiol 33:1225–1228
Jørgensen PE, Eriksen T, Jensen BK (1992) Estimation of viable biomass in wastewater and activated sludge by determination of ATP, oxygen utilization rate and FDA hydrolysis. Water Res 26:1495–1501
Lessie TJ, Vander Wyk JC (1972) Multiple forms of Pseudomonas multivorans glucose-6-phosphate and 7-phosphogluconate dehydrogenases: differences in size, pyridine specificity and susceptibility to inhibition by adenosine 5′-triphosphate. J Bacteriol 110:1107–1117
Li D, Ganzarczyk JJ (1990) Structure of activated sludge flocs. Biotechnol Bioeng 35:57–65
Meyer-Reil l-A (1992) Ecological aspects of enzymatic activity in marine sediments. In: Chróst RJ (ed) Microbial enzymes in aquatic environments. Springer, New York Berlin Heidelberg, p 85
Nielsen PH, Raunkjær K, Norsker NH, Jensen NAa, Hvitved-Jacobsen T (1992) Transformation of wastewater in sewer systems—a review. Water Sci Tech 25:17–31
Nybroe O, Jørgensen PE, Hence M (1992) Enzyme activities in wastewater and activated sludge. Water Res 26:579–584
Obst U (1985) Test instructions for measuring the microbial activity in water samples. Fresenium Z, Anal Chem 321:166–168
Peterson GL (1979) Review of the folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem 100:201–219
Randtke SJ, Larson RA (1983) Comment. Water Res 18:1597–1599
Raunkjær K, Hvitved-Jacobsen T, Nielsen PH (1994) Measurements of pools of protein, carbohydrate and lipid in domestic wastewater. Water Res 28:251–262
Richards SR, Hastwell C, Davies M (1984) The comparative examination of 14 activated-sludge plants using enzymatic techniques. Water Pollut Control 83:300–313
Riffaldi R, Sartori F, Levi-Minzi R (1982) Humic substances in sewage sludges. Environ Pollut 3:139–146
Rudd T, Sterrit RM, Lester JW (1983) Extraction of extracellular polymers from activated sludge. Biotechnol Lett 5:327–332
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as an estimator of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Swisher R, Carroll GC (1980) Fluorescein diacetate hydrolysis as an estimator of microbial biomass on coniferous needle surfaces. Microb Ecol 6:217–226
Teuber M, Brodisch KEU (1977) Enzymatic activities of activated sludge. Euro J Appl Microbiol 4:185–194
Urbain V, Block JC, Manem J (1993) Bioflocculation in activated sludge: an analytical approach. Water Res 27:829–838
Wetzel RG (1991) Extracellular enzymatic interactions: storage, redistribution and interspecific communication. In: Chrøst RJ (ed) Microbial enzymes in aquatic environments. Springer, New York Berlin Heidelberg, p 6
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fr/olund, B., Griebe, T. & Nielsen, P.H. Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol 43, 755–761 (1995). https://doi.org/10.1007/BF00164784
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164784