Summary
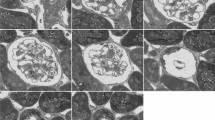
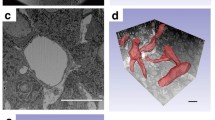
Several recent studies comparing chemically fixed and cryofixed endothelium have indicated that glutaraldehyde fixation may result in increases in the population of “vesicles” in the cytoplasm. Other reports based on ultrathin serial-section reconstruction of chemically fixed endothelium have revealed that the vesicular system is comprised of interconnected membranous compartments, which are ultimately continuous with either cell surface but do not extend across the endothelial cell. In this study, we have investigated the three-dimensional organization of the vesicular system in directly frozen, freeze-substituted capillaries of the rete mirabile from the swim bladder of the eel, specifically using the same block of embedded capillaries in which frozen capillaries had previously been found to contain less “vesicles” than chemically fixed capillaries. The results show that essentially all vesicles remain inter-connected with each other and are part of two separate sets of invaginations from the luminal and abluminal cell surface like in chemically fixed tissue. Any increase in vesicle number resulting from glutaraldehyde fixation does not affect the overall three-dimensional organization of the vesicular system in these endothelial cells.
Similar content being viewed by others
References
Bridgman PC, Reese TS (1984) The structure of cytoplasm in directly frozen cultured cells. I. Filamentous meshworks and the cytoplasmic ground substance. J Cell Biol 99:1655–1668
Buchanan R, Wagner RC, Andrews BS, Frøkjær-Jensen J (1988) Effect of section thickness on the morphological characterization of the vesicular system in endothelial cells. Microvasc Res (in press)
Bundgaard M (1987) Tubular invaginations in cerebral endothelium and their relation to smooth-surfaced cisternae in hagfish (Myxine glutinosa). Cell Tissue Res 249:359–365
Bundgaard M, Frøkjær-Jensen J, Crone C (1979) Endothelial plasmalemmal vesicles as elements in a system of branching invaginations from the cell surface. Proc Natl Acad Sci USA 76:6439–6442
Bundgaard M, Hagman P, Crone C (1983) The three-dimensional organization of plasmalemmal vesicular profiles in the endothelium of rat heart capillaries. Microvasc Res 25:358–368
Casley-Smith JR (1981) Freeze-substitution observations on the endothelium, and the passage of ions. Microcirculation 1:79–109
Frøkjær-Jensen J (1980) Three-dimensional organization of plasmalemmal vesicles in endothelial cells. An analysis by serial sectioning of frog mesenteric capillaries. J Ultrastruct Res 73:9–20
Frøkjær-Jensen J (1983) The plasmalemmal vesicular system in capillary endothelium. Conventional electron microscopic (EM) thin sections compared with the picture arising from ultrathin (≈140 Å) serial sectioning. Prog Appl Microcirc 1:17–34
Frøkjær-Jensen J (1984) The plasmalemmal vesicular system in striated muscle capillaries and in pericytes. Tissue Cell 16:31–42
Frøkjær-Jensen J (1985) The continuous capillary. Structure and Function. Biol Skr Dan Vid Selsk 25:209–253
Frøkjær-Jensen J, Reese TS (1985) Three-dimensional organization of the plasmalemmal vesicular system in directly frozen, freezesubstituted frog mesenteric capillaries. J Physiol (London) 371:84P [Abstract]
Haraldsson B, Johansson BR (1985) Changes in transcapillary exchange induced by perfusion fixation with glutaraldehyde, followed by measurements of capillary filtration coefficient, diffusion capacity and albumin clearance. Acta Physiol Scand 124:99–106
Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L (1979) Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol 81:275–300
Jennings MA, Florey L (1967) An investigation of some properties of endothelium related to capillary permeability. Proc R Soc Lond [Biol] 167:39–63
Kobayashi S (1970) Ferritin labeling in the fixed muscle capillary. A doubt on the tracer-experiments as the basis for the vesicular transport theory. Arch Histol Jpn 32:81–86
Mazzone RW, Kornblau SM (1981) Pinocytotic vesicles in the endothelium of rapidly frozen rabbit lung. Microvasc Res 21:193–211
McGuire PG, Twietmeyer TA (1983) Morphology of rapidly frozen aortic endothelial cells. Glutaraldehyde fixation increases the number of caveolae. Circ Res 53:424–429
Nagy Z, Pettigrew KD, Meiselman S, Brightman MW (1987) Cerebral vessels cryofixed after hyperosmosis or cold injury in normothermic and hypothermic frogs. Brain Res (in press)
Noguchi Y, Shibata Y, Yamamoto T (1987) Endothelial vesicular system in rapid-frozen muscle capillaries revealed by serial sectioning and deep etching. Anat Rec 217:355–360
Palade GE (1953) Fine structure of blood capillaries. J Appl Phys 24:1424 [Abstract]
Palade GE, Bruns RR (1968) Structural modulations of plasmalemmal vesicles. J Cell Biol 37:633–649
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–213
Rippe B, Kamiya A, Folkow B (1979) Transcapillary passage of albumin, effects of tissue cooling and of increases in filtration and plasma colloid osmotic pressure. Acta Physiol Scand 105:171–187
Rippe B, Haraldsson B (1986) Capillary permeability in rat hindquarters as determined by estimations of capillary reflection coefficients. Acta Physiol Scand 127:289–303
Robinson JM, Hoover RL, Karnovsky MJ (1984) Vesicle (caveolae) number is reduced in cultured endothelial cells prepared for electron microscopy by rapid-freezing. J Cell Biol 99:287a [Abstract]
Simionescu M, Simionescu N (1984) Ultrastructure of the microvascular wall: functional correlations. In: Renkin EM, Michel CC (eds) Handbook of Physiology vol IV, The Cardiovascular System. American Physiological Society, Washington, USA, pp 41–101
Simionescu N, Simionescu M, Palade GE (1975) Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol 64:586–607
Taylor AE, Granger DN (1984) Exchange of macromolecules across the circulation. In: Renkin EM, Michel CC (eds) Handbook of Physiology, vol IV, The Cardiovascular System. American Physiological Society, Washington, USA, pp 467–520
Wagner RC, Andrews SB (1985) Ultrastructure of the vesicular system in rapidly frozen capillary endothelium of the rete mirabile. J Ultrastruct Res 90:172–182
Wagner RC, Robinson CS (1984) High-voltage electron microscopy of capillary endothelial vesicles. Microvasc Res 28:197–205
Wolff JR (1967) On the meaning of vesiculation in capillary endothelium. Angiologica 4:64–68
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frøkjær-Jensen, J., Wagner, R.C., Andrews, S.B. et al. Three-dimensional organization of the plasmalemmal vesicular system in directly frozen capillaries of the rete mirabile in the swim bladder of the eel. Cell Tissue Res. 254, 17–24 (1988). https://doi.org/10.1007/BF00220012
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220012