Summary
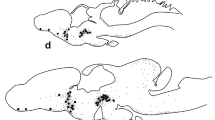
Immunohistochemical double staining for gonadotropin releasing hormone (GnRH) and tyrosine hydroxylase (TH) or glutamic acid decarboxylase (GAD) reveals in the septo-preopticdiagonal band complex of the rat brain close spatial associations between GnRH-immunoreactive perikarya and TH and GAD immunoreactive fibers. In the organum vasculosum laminae terminalis, no close spatial relationships could be observed between TH-or GAD-positive fibers and the GnRH-containing system. In contrast, in the median eminence substantial overlap exists in the distribution of GnRH with TH and GAD containing nerve fibers. This overlap is most intense for TH throughout the lateral palisade zone, while for GAD it is more restricted to the outermost portion of the external palisade zone. The results suggest that dopamine and GABA influence GnRH secretion via axosomatic contacts in the septo-preoptic-diagonal band complex, as well as via axo-axonic interactions in the median eminence, while no such interactions seem to exist in the organum vasculosum laminae terminalis. Since dopaminergic cell bodies in the ventral hypothalamus are closely apposed by GnRH and GAD containing fibers, the existence of feedback circuits among GnRH, dopamine and GABA systems is proposed.
Similar content being viewed by others
References
Barraclough CA, Wise PM (1982) The role of catecholamines in the regulation of pituitary luteinizing hormone and folliclestimulating hormone secretion. Endocrinol Rev 3: 91–119
Barry J (1979) Immunohistochemistry of luteinizing hormonereleasing hormone-producing neurons of the vertebrates. Int Rev Cytol 60: 179–221
Beck W, Hancke J, Wuttke W (1978) Increased sensitivity of dopaminergic inhibition of luteinizing hormone release in immature and castrated female rats. Endocrinology 102: 837–843
Bennett GW, Edwardson JA, Holland D, Jeffcoate SL, White N (1975) Release of immunoreactive luteinizing-hormone-releasing hormone and thyrotrophin-releasing hormone from hypothalamic synaptosomes. Nature 257: 323–325
Björklund A, Nobin A (1973) Fluorescence histochemical and microspectrofluorometric mapping of dopamine and noradrenaline cell groups in the rat diencephalon. Brain Res 51: 193–205
Conn PM, Marian J, McMillian M, Stern J, Rogers D, Hamby M, Penna A, Grant E (1981) Gonadotropin releasing hormone action in the pituitary: A three step mechanism. Endocrinol Rev 2: 174–185
Fuxe K, Andersson K, Ogren S-O, Perez De La Mora M, Schwarcz R, Hökfelt T, Eneroth P, Gustafsson J-A, Skett P (1978) GABA neurons and their interaction with monoamine neurons. An anatomical, pharmacological and functional analysis. In: GABA Neurotransmitters, Alfred Benzon Symposium 12. Munksgaard, Copenhagen, pp 74–94
Gallo RV, Osland RB (1976) Electrical stimulation of the arcuate nucleus in ovariectomized rats inhibits episodic luteinizing hormone (LH) releast but excites LH relase after estrogen priming. Endocrinology 99: 659–668
Hery M, Laplante E, Kordon C (1976) Participation of serotonin in the phasic release of LH. I. Evidence from pharmacological experiments. Endocrinology 99: 496–503
Hökfelt T, Johansson O, Fuxe K, Goldstein M, Park D (1976) Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I. Tyrosine hydroxylase in the mes- and diencephalon. Med Biol 54: 427–453
Hökfelt T, Elde R, Fuxe K, Johansson O, Ljungdahl A, Goldstein M, Luft R, Efendic S, Nilsson G, Terenius L, Ganten D, Jeffcoate SL, Rehfeld J, Said S, Perez De La Mora M, Possani L, Tapia R, Teran L, Palacios R (1978) Aminergic and peptidergic pathways in the nervous system with special reference to the hypothalamus. In: Reichlin S, Baldessarni RJ, Martin JB (eds) The hypothalamus. Raven Press, New York, pp 69–133
Jennes L, Stumpf WE (1980) LHRH-systems in the brain of the golden hamster. Cell Tissue Res 209: 239–256
Jennes L, Beckman WC, Stumpf WE, Grzanna R (1982) Anatomical relationships of serotonergic and noradrenalinergic projections with the GnRH system in septum and hypothalamus. Exp Brain Res 46: 331–338
Joh TH, Gregham G, Reis DJC (1973) Immunochemical demonstration of increased tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci USA 70: 2767–2771
Löfstrom A (1977) Brain catecholamines and the control of ovulation. Civiltryck, Stockholm, p 55
Mansky T, Wuttke W (1981) Involvement of GABA in negative feedback action of estradiol. In: Wuttke W, Horowski R (eds) Gonadal steroids and brain function. Springer, Berlin Heidelberg New York, pp 366–369
Negro-Vilar A, Ojeda SR, McCann SM (1979) Catecholaminergic modulation of luteinizing hormone-releasing hormone release by median eminence terminals in vitro. Endocrinology 104: 1748–1757
Negro-Vilar A, Ojeda SR, McCann SM (1980) Hypothalamic control of LHRH and somatostatin: Role of central neurotransmitters and intracellular messengers. In: Litwack G (ed) Biochemical actions of hormones. Academic Press, New York, pp 245–285
Oertel WH, Schmechel DE, Tappaz ML, Kopin LJ (1981) Production of a specific antiserum to rat brain glutamic acid decarboxylase (GAD) by injection of an antigen-antibody complex. Neuroscience 6: 2689–2700
Palkovits M, Fekete M, Makara GB, Herman JP (1977) Total and partial hypothalamic deafferentations for topographical identification of catecholaminergic innervations of certain preoptic and hypothalamic nuclei. Brain Res 127: 127–136
Pass KA, Ondo JG (1977) The effects of gamma-aminobutyric acid on prolactin and gonadotropin secretion in the unanesthetized rat. Endocrinology 100: 1437–1442
Sawyer CH, Markee JE, Hollinshead WH (1947) Inhibition of ovulation in the rabbit by the adrenergic-blocking agent Dibenamine. Endocrinology 41: 395–402
Sawyer CH, Clifton DK (1980) Aminergic innervation of the hypothalamus. Fed Proc 39: 2889–2895
Tappaz ML, Wassef M, Oertel WH, Paul L, Pujol JF (1983) Light- and electronmicroscopic immunohistochemistry of glutamic acid decarboxylase (GAD) in the basal hypothalamus: Morphological evidence for neuroendocrine GABA. Neuroscience (in press)
Tappaz ML, Oertel WH, Wassef M, Mugnaini E (1983) Central GABAergic neuroendocrine regulations: Pharmacological and morphological evidence. Prog Brain Res (in press)
Vijayan E, McCann SM (1978) The effects of intraventricular injection of gamma-aminobutyric acid (GABA) on prolactin and gonadotropin release in conscious female rats. Brain Res 155: 35–43
Weiner RI, Ganong WF (1978) Role of brain monoamines and histamine in regulation of anterior pituitary. Physiol Rev 58: 905–976
Wuttke W, Mansky T (1981) Gonadal steroids and brain monoamines: How do they interact? In: Wuttke W, Horowski R (eds) Gonadal steroids and brain function. Springer, Berlin Heidelberg New York, pp 130–141
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jennes, L., Stumpf, W.E. & Tappaz, M.L. Anatomical relationships of dopaminergic and GABAergic systems with the GnRH-systems in the septo-hypothalamic area. Exp Brain Res 50, 91–99 (1983). https://doi.org/10.1007/BF00238235
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00238235