Summary
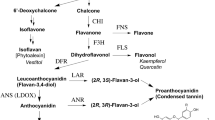
Chemogenetic investigations and precursor experiments on flowers of Petunia hybrida suggest that recessive alleles of the gene An3 block the biosynthetic pathway of flavonols and anthocyanins between the flavanone and dihydroflavonol step. In confirmation of this hypothesis, activity of the enzyme flavanone 3-hydroxylase, which catalyses the conversion of flavanones to dihydroflavonols, was readily demonstrated in enzyme preparations from flowers of lines with the dominant allele An3, whereas no or very low activity could be found in extracts from lines with recessive alleles (an3an3). A second genetic factor is described which clearly reduces the amount of flavonols in the flowers but not the amount of anthocyanins. Crossing experiments revealed that this factor represents a third allele of the An3 gene. It is referred to as an3-1. As expected, a residual flavanone 3-hydroxylase activity of about 10% could be found in enzyme extracts from plants with the an3-1 allele. The decreased level of dihydroflavonol formed under this condition is obviously still sufficient for anthocyanin formation but not for flavonol synthesis.
Similar to flavanone 3-hydroxylases from other plants, the enzyme of Petunia is a soluble enzyme and belongs according to its cofactor requirements to the 2-oxoglutarate-dependent dioxygenases. The residual flavanone 3-hydroxylase activity found in plants with the an3-1 allele is identical to the activity extracted from An3-genotypes with regard to cofactors, substrate specificity and most of the inhibitors. The difference observed in the respective pH-optima and the genetic data suggest that the mutation providing the an3-1 phenotype is localized in the structural gene for flavanone 3-hydroxylase.
Similar content being viewed by others
References
Abbott MT, Udenfriend S (1974) α-ketoglutarate-coupled dioxygenases. In: Hayaishi O (ed) Molecular mechanisms of oxygen activitation. Academic Press, New York London, pp 167–214
Barton GM (1968) Detection of 3-hydroxylflavanones on papergrams and thin-layer plates. J Chromatogr 34:562
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britsch L, Heller W, Grisebach H (1981) Conversion of flavanone to flavone, dihydroflavonol and flavonol with an enzyme system from cell cultures of parsley. Z Naturforsch 36:742–750
Doodeman M, Tabak AJH, Schram AW, Bennink GJH (1982) Hydroxylation of cinnamic acids and flavonoids during biosynthesis of anthocyanins in Petunia hybrida hort. Planta 154:546–549
Eigen E, Blitz M, Gunsberg E (1957) The detection of some naturally occuring flavanone compounds on paper chromatograms. Arch Biochem Biophys 68:501
Forkmann G, Heller W, Grisebach H (1980) Anthocyanin biosynthesis in flowers of Matthiola incana. Flavanone 3- and flavonoid 3′-hydroxylases. Z Naturforsch 35:691–695
Forkmann G, Stotz G (1981) Genetic control of flavanone 3-hydroxylase activity and flavonoid 3′-hydroxylase activity in Antirrhinum majus (Snapdragon). Z Naturforsch 36:411–416
Forkmann G, Stotz G (1984) Selection and characterisation of flavanone 3-hydroxylase mutanls of Dahlia, Streptocarpus, Verbena and Zinnia. Planta 161:261–265
Gerats AGM, de Vlaming P, Doodeman M, Al B, Schram AW (1982) Genetic conlrol of the conversion of dihydroflavonols into flavonols and anthocyanins in flowers of Petunia hybrida. Planla 155:364–268
Kho KFF, Bolsman-Louwen AC, Vuik JC, Bennink GJH (1977) Anthocyanin synthesis in a white flowering mutant of Petunia hybrida. Planla 135:109–118
Maizonnier D, Moessner A (1979) Localisation of the linkage groups on the seven chromosome of the Petunia hybrida genome. Genetics 51:143–148
Spribille R, Forkmann G (1984) Conversion of dihydroflavonols to flavonols with enzyme exlracts from flower buds of Matthiola incana R. Br. Z Nalurforsch 39:714–719
Stotz G (1983) Enzymologie und Genetik der Oxidalionsreaklionen in der Flavonoid-Biosynthese höherer Pflanzen. Ph D Thesis, Universität Tübingen (FRG)
Stotz G, de Vlaming P, Wiering H, Schram AW, Forkman G (1985) Genetic and biochemical sludies on flavonoid 3′-hydroxylalion in flowers of Petunia hybrida. Theor Appl Genet 70:300–305
Tabak AJH, Meyer H, Bennink GJH (1978) Modification of the B-ring during flavonoid synlhesis in Petunia hybrida: introduction of the 3′-hydroxyl group regulated by the gene Ht1. Planta 139:67–71
Wiering H (1974) Genetics of flower colour in Petunia hybrida hort. Genen Phaenen 17:117–134
Wiering H, de Vlaming P, Cornu A, Maizonnier D (1979) Petunia genetics. 1. List of genes. Ann Amelior Plant 29:611–622
Author information
Authors and Affiliations
Additional information
Communicated by D. von Wettstein
Rights and permissions
About this article
Cite this article
Froemel, S., de Vlaming, P., Stotz, G. et al. Genetic and biochemical studies on the conversion of flavanones to dihydroflavonols in flowers of Petunia hybrida . Theoret. Appl. Genetics 70, 561–568 (1985). https://doi.org/10.1007/BF00305991
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305991