Abstract
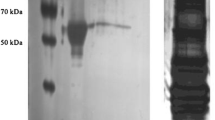
Glycogen phosphorylase (EC 2.4.1.1) of Manduca sexta flight muscle was separated into three distinct peaks of activity on diethylaminoethyl-Sephacel. The three fractions of phosphorylase activity were further purified by affinity chromatography on AMP-Sepharose and shown to have the same relative molecular mass (=178000) on polyacrylamide gradient gel electrophoresis under non-denaturating conditions and to produce subunits of molecular mass =92000 on SDS gelelectrophoresis. On the basis of their kinetic properties with respect to the activator AMP and the inhibitor caffeine, the three fractions of phosphorylase activity were assigned as follows: peak 1=phosphorylase b (unphosphorylated form), peak 3=phosphorylase a (phosphorylated form); peak 2 represented a phospho-dephospho hybrid in which only one subunit of the dimeric enzyme was phosphorylated. This hypothesis was corroborated as the various forms could be interconverted in vitro by either dephosphorylation by an endogenous protein phosphatase producing the b form, or by phosphorylation catalyzed by purified phosphorylase kinase from rabbit muscle producing phosphorylase ab and a. From muscle of resting moths more phosphorylase was isolated in the b form (41%) than in the forms ab (28%) and a (31%), respectively. This proportion was changed in favour of the fully phosphorylated a form after a brief interval of flight when 68% of the phosphorylase activity was represented by the a form and only 13% by the b form. Unlike the phosphorylated forms a and ab of phosphorylase, the b form had low affinities for the substrates and for the activator AMP, and was virtually inactive if near-physiological concentrations of substrates and effectors were employed in the assays. The results demonstrate that in Manduca flight muscle three forms of phosphorylase coexist and that their interconversion is a mechanism for regulating phosphorylase activity in vivo.
Similar content being viewed by others
Abbreviations
- DEAE:
-
diethylaminoethyl
- EDTA:
-
ethylenediamine tetraacetate
- EGTA:
-
ethyleneglycol-bis(β-aminoethylether)N,N′-tetra-acetic acid
- M r :
-
relative molecular mass
- NMR:
-
nuclear magnetic resonance
- PAGGE:
-
polyacrylamide gradient gel electrophoresis
- Pi :
-
morganic phosphate
- SDS:
-
sodium dodecylsulphate
- TRIS:
-
tris(hydroxymethyl)-aminomethane
- V max :
-
maximum activity
References
Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJA (1984) Insect flight muscle metabolism. Insect Biochem 14:243–260
Bell RA, Joachim FG (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am 69:365–373
Busby SJW, Radda GK (1976) Regulation of the glycogen phosphorylase system-from physical measurements to biological speculations. Curr Top Cell Regul 10:90–160
Candy DJ (1989) Utilization of fuels by the flight muscles. In: Goldsworthy GJ, Wheeler CH (eds) Insect flight. CRC Press, Boca Raton, pp 305–319
Childress CC, Sacktor B (1970) Regulation of glycogen metabolism in insect flight muscle. Purification and properties of phosphorylases in vitro and in vivo. J Biol Chem 245:2927–2936
Dombradi V (1981) Structural aspects of the catalytic and regulatory function of glycogen phosphorylase. Int J Biochem 13:125–139
Downer RGH, Parker GH (1979) Glycogen utilisation during flight in the American cockroach, Periplaneta americana L. Comp Biochem Physiol 64A:29–32
Fischer EH, Heilmeyer LMG, Haschke RH (1971) Phosphorylase and the control of glycogen degradation. Curr Top Cell Regul 4:211–249
Gäde G (1988) Das Phosphorylasesystem im Fettkörper von Schaben: Regulation mittels hypertrehalosämischer Hormone. Verh Dtsch Zool Ges 81:309
Gäde G (1991) Glycogen phosphorylase in the fat body of two cockroach species, Periplaneta americana and Nauphoeta cinerea: isolation, partial characterization of three forms and activation by hypertrehalosaemic hormones. Z Naturforsch 46c:149–162
Gergely P, Bot G, Kovács EF (1974) Partial phosphorylation of muscle phosphorylase. II. Formation of a hybrid phosphorylase in vivo. Biochim Biophys Acta 370:78–84
Heilmeyer LMG Jr, Meyer F, Haschke RH, Fischer EH (1970) Control of phosphorylase activity in a muscle glycogen particle. II. Activation by calcium. J Biol Chem 245:6649–6656
Helmreich E, Michaelides MC, Cori CF (1967) Effects of substrates and a substrate analog on the binding of 5′-adenylic acid to muscle phosphorylase a. Biochemistry 6:3695–3710
Hisamitsu S (1979) Improved disc gel electrophoresis for detecting glycogen phosphorylase isozymes. Acta Histochem Cytochem 12:47–55
Krebs EG (1981) Phosphorylation and dephosphorylation of glycogen phosphorylase: a prototype for reversible covalent modification. Curr Top Cell Regul 18:401–419
Krebs EG, Fischer EH (1962) Molecular properties and transformations of glycogen phosphorylase in animal tissues. Adv Enzymol Relat Areas Mol Biol 14:263–290
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Madsen NB (1986) Glycogen phosphorylase. In: Boyer PD, Krebs EG (eds) The enzymes, 3rd edn, vol 17. Academic Press, Orlando New York London, pp 365–394
Rowan AN, Newsholme EA (1979) Changes in the contents of adenine nucleotides and intermediates of glycolysis and the citric acid cycle in flight muscle of the locust upon flight and their relationship to the control of the cycle. Biochem J 178:209–216
Schmidt H, Wegener G (1990) Glycogen phosphorylase in fish muscle: demonstration of three interconvertible forms. Am J Physiol 258 (Cell Physiol 27):C344-C351
Sprang SR, Acharya KR, Goldsmith EJ, Stuart DI, Varvill K, Fletterick RJ, Madsen NB, Johnson LN (1988) Structural changes in glycogen phosphorylase induced by phosphorylation. Nature 336:215–221
Steele JE (1982) Glycogen phosphorylase in insects. Insect Biochem 12:131–147
Vaandrager SH, Van Marrewijk WJA, Beenakkers AMT (1986) Glycogen phosphorylase activity in flight muscles of Locusta migratoria at rest and during flight. Insect Biochem 16:749–756
Van Marrewijk WJA, Van den Broek ATM, Beenakkers AMT (1985) Glycogen phosphorylase in the fat body of Locusta migratoria. Occurrence of three interconvertible forms. Insect Biochem 15:341–347
Van Marrewijk WJA, Van den Broek ATM, Beenakkers AMT (1989) Occurrence of three forms of glycogen phosphorylase and flight-induced enzyme activation in fat body of the American cockroach, Periplaneta americana. Comp Biochem Physiol 94B:165–169
Vereb G, Fodor A, Bot G (1987) Kinetic characterization of rabbit skeletal muscle phosphorylase ab hybrid. Biochim Biophys Acta 915:19–27
Wegener G, Beinhauer I, Klee A, Newsholme EA (1987) Properties of locust muscle 6-phosphofructokinase and their importance in the regulation of glycolytic flux during prolonged flight. J Comp Physiol B 157:315–326
Wegener G, Bolas NM, Thomas AAG (1991) Locust flight metabolism studied in vivo by 31P NMR spectroscopy. J Comp Physiol B 161:247–256
Wollenberger A, Ristau O, Schoffa G (1960) Eine einfache Technik der extrem schnellen Abkühlung größerer Gewebestücke. Pflügers Arch Ges Physiol 270:399–412
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burkhardt, G., Wegener, G. Glycogen phosphorylase from flight muscle of the hawk moth, Manduca sexta: purification and properties of three interconvertible forms and the effect of flight on their interconversion. J Comp Physiol B 164, 261–271 (1994). https://doi.org/10.1007/BF00346441
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00346441