Abstract
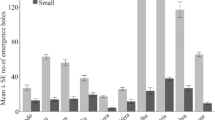
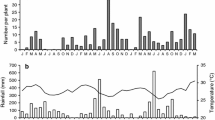
Cherry trees (Prunus serotina) responded to oviposition by periodical cicadas (Magicicada spp.) by depositing gum at the egg nest. The proportion of cicada eggs that hatched successfully was significantly reduced at egg nests with visible gum compared to non-gummed egg nests. The number of egg nests with gum increased in proportion to the total number of egg nests on a tree. The probability of an egg nest having visible gum increased as the total number of egg nests increased. Mortality at hatching due to gum deposition increased as a direct density-dependent function of the number of cicada eggs laid in the tree. Although statistically significant, this relationship was weak and appeared to hold only at densities above 100 egg nests per tree.
Gum deposition is discussed as an induced plant response to cicada attack. A cherry may reduce the number of cicada nymphs that will parasitize it up to the next oviposition period (17 or 13 years later) by reducing cicada hatching through gum deposition at the site of oviposition.
Similar content being viewed by others
References
Banta ES (1960) Apple orchard decline. Proc Ohio Hortic Soc 118:88–90
Benz G (1977) Insect induced resistance as a means of self defense. International organization for biological control of noxious animals and plants. Bulletin SROP 1977:155–159
Bryant JP (1981) Phytochemical deterrence of snowshoe hare browsing by adventitious shoots of four Alaskan trees. Science 213:889–890
Butler AW (1886) The periodical cicada. Bull USDA Div Entomol 12:24–31
Butler O (1911) A study on gummosis of Prunus and Citrus with observations on squamosis and exanthema of the Citrus. Ann Bot 25:107–154
Cory EN, Knight P (1937) Observations on brood X of the periodical cicada in Maryland. J Econ Ent 30:287–294
Dybas HS, Davis DD (1962) A population census of seventeen-year periodical cicadas (Homoptera: Cicadidae: Magicicada). Ecology 43:432–444
Dybas HS, Lloyd M (1974) The habitats of 17-year periodical cicadas (Homoptera: Cicadidae: Magicicada spp.). Ecol Monogr 44:279–324
Esau K (1965) Plant anatomy, Ed 2. John Wiley, New York
Feigl F, Anger V (1966) Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst 91:282–284
Forsythe HY (1976) Number of seventeen-year cicada eggs per nest. Environ Ent 5:169–170
Freeland WJ (1974) Vole cycles — another hypothesis. Am Nat 108:238–245
Graham C, Cochran AB (1954) The periodical cicada in Maryland in 1953. J Econ Ent 47:242–244
Hamilton DW (1961) Periodical cicadas, Magicicada spp., as pests in apple orchards. Proc Indiana Acad Sci 71:116–121
Hamilton DW, Cleveland ML (1964) Periodical cicadas in 1963, Brood 23. Proc Indiana Acad Sci 73:167–170
Harris P (1960) Production of pine resin and its effect on survival of Rhyacionia buoliana (Schiff.) (Lepidoptera: Olethreutidae). Can J Zool 38:121–131
Haukioja E (1980) On the role of plant defenses in the fluctuation of hervivore populations. Oikos 35:202–213
Haukioja E, Hakala T (1975) Herbivore cycles and periodic outbreaks. Formulation of a general hypothesis. Rep kevo Subarctic Res Station 12:1–9
Hopkins AD (1897) The periodical cicada. W Va Agric Exp Sta Bull 68:1–46
Hunter PE, Lund HO (1960) Biology of the periodical cicada in Georgia. J Econ Ent 53:961–963
Karban R (1980) Periodical cicada nymphs impose periodical oak tree wood accumulation. Nature 287:326–327
Karban R (1981) Flight and dispersal of periodical cicadas. Oecologia (Berlin) 49:385–390
Karban R (1982) Experimental removal of 17-year cicada nymphs and growth of host apple tress. J NY Ent Soc 90:74–81
Langenheim JH, Arrhenius SP, nascimento JC (1981) Relationship of light intensity to leaf resin composition and yield in the tropical leguminous genera Hymenaea and Copaifera. Biochem System Ecol 9:27–37
Levin DA (1976) The chemical defenses of plants to pathogens and herbivores. Ann Rev Ecol Syst 7:121–160
Lloyd M, Dybas HS (1966) The periodical cicada problem. I. Population ecology. II. Evolution Evolution 20:133–149, 466–505
Lloyd M, Karban R (1983) Chorusing centers of periodical cicadas. J Kansas Ent Soc (in press)
Lloyd M, White J (1976) On the oviposition habits of 13-year versus 17-year periodical cicadas of the same species. J NY Ent Soc 84:148–155
Marlatt CL (1907) The periodical cicada. Bull USDA Bur Ent 71:1–181
Metcalf CL, Flint WP, Metcalf RL (1962) Destructive and useful insects: Their habits and control McGraw-Hill, New York
Olien WC, Bukovac MA (1982) Ethopon-induced gummosis in sour cherry (Prunus cerasus L.) I. Effect on xylem function and shoot water status. Plant Physiol 70:547–555
Reid RW, Whitney HS, Watson JA (1967) Reactions of lodgepole pine to attack by Dendroctonus ponderosae Hopkins and blue stain fungi. Can J Bot 45:1115–1126
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores: Their inteaction with secondary plant metabolites. Academic Press, New York
Riley CV (1885) The periodical cicada. An account of Cicada septendecim and its tredecim race, with a chronology of all broods known. Bull USDA Div Ent 8:1–46
Schlicting CD (1983) Wound-induced defenses of plants and counteradaptation by herbivores. Oikos (in press)
Schultz JC, Baldwin IT (1982) Oak leaf quality decline in response to defoliation by gypsy moth larvae. Science 217:149–151
Simon CM, Karban R, Lloyd M (1981) Patchiness, density and aggregative behavior in sympatric allochronic populations of 17-year cicadas. Ecology 62:1525–1535
Smith F, Montgomery R (1959) The chemistry of plant gums and mucilages and some related polysaccharides. Reinhold, New York
Smith FF, Linderman RG (1974) Damage to ornamental trees and shrubs resulting from oviposition by periodical cicadas. Env Ent 3:725–732
Stark RW (1965) Recent trends in forest entomology. Ann Rev Entom 10:303–324
Talboys PW (1968) Water deficits in vascular disease: In: Kozlowski TT (ed) Water deficits and plant growth (Vol II)
Wallner WE, Walton GS (1979) Host defoliation: a possible determinant of gypsy moth population quality. Ann Ent Soc Amer 72:62–67
White J (1973) Viable hybrid young from crossmated periodical cicadas. Ecology 54:573–580
White J (1980) Resource partitioning by ovipositing cicadas. Amer Nat 115:1–28
White J (1981) Flagging: hosts defenses versus oviposition strategies in periodical cicadas (Magicicada spp., Cicadidae, Homoptera). Can Ent 113:727–738
White J, Lloyd M (1975) Growth rates of 17- and 13-year periodical cicadas. Am Midl Nat 94:127–143
White J, Lloyd M (1981) On the stainability and mortality of periodical cicada eggs. Am Midl Nat 106:219–228
White J, Strehl CE (1978) Xylem feeding by periodical cicada nymphs on tree roots. Ecol Ent 3:323–327
Wood DL (1973) Selection and colonization of ponderosa pine by bark beetles. In: Insect/plant relationships. Symposia of the Royal Ent Soc London 6:101–118
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karban, R. Induced responses of cherry trees to periodical cicada oviposition. Oecologia 59, 226–231 (1983). https://doi.org/10.1007/BF00378841
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00378841