Abstract
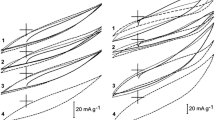
The possibility of removing hexavalent chromium from waste water by electrochemical treatment using a graphite felt electrode and synthetic electrolytes is investigated. It is suggested that the process proceeds in two steps: electrochemical reduction of the hexavalent chromium to chromic ion followed by the formation of an insoluble chromic hydroxide in an electrochemically generated high pH environment. The chromic hydroxide adheres to the electrode surface as a charged colloidal particle. The electrochemical dissolution of the hydroxide layer by potential inversion is also discussed as a possible regeneration procedure.
It is concluded that these steps can be included within a single separating column provided that the feed pH and the column voltage are carefully controlled.
Similar content being viewed by others
References
B. W. Morris, C. A. Hardisty, J. F. McCann, G. J. Kamp and T. W. May,Atomic Spectrosc. 6 (1985) 149.
R. A. Anderson, M. M. Polansky, N. A. Bryden, K. Y. Patterson, C. Veillon and W. H. Glinsmann,J. Nutrit. 113 (1983) 276.
‘International Standards for Drinking Water,’ 3rd edn, WHO, Geneva (1971).
‘European Standards for Drinking Water,’ 2nd edn, WHO Regional Office for Europe, Copenhagen (1970).
T. T. Taylor,Chem. Eng. Progr. 79 (1982) 70.
P. P. Bronovolokov and V. V. Pushkarev,Zh. Prikl. Khim. (Leningrad) 51 (1978) 1863.
J. Bishop,Chem. Week 11 February (1987) 14.
C. P. Huang and M. H. Wu,Water Res. 11 (1977) 673.
I. C. Agarwal, A. M. Rochon, H. D. Gesser and A. B. Sparling,Water Res. 18 (1984) 227.
F. A. Lowenheim (Ed.), ‘Modern Electroplating,’ Chapman and Hall, London (1974).
F. Ogburn and A. Brenner,J. Electrochem. Soc. 96 (1949) 347.
J. P. Hant and H. Taub,J. Chem. Phys. 19 (1951) 602;18 (1950) 757.
J. P. Hoare,J. Electrochem. Soc. 126 (1979) 190.
K. Nishimura, H. Fukushima, T. Akiyama and K. Higashi,Metal Finish. March (1987) 45.
J. Lin-Cai and D. Pletcher,J. Appl. Electrochem. 13 (1983) 245.
K. Othmer, ‘Encyclopedia of Chemical Technology,’ Interscience Publishers, New York (1964) Vol. 5, p. 842.
Y. Oren and A. Soffer,Electrochim. Acta 28 (1983) 1649.
C. Zur and M. Ariel,J. Appl. Electrochem. 11 (1981) 639.
A. T. Hubbard,J. Electroanal. Chem. 22 (1969) 165.
A. T. Hubbard and F. C. Anson, in ‘Electroanalytical Chemistry,’ Vol. 5 (edited by A. J. Bard), Dekker, New York (1971).
J. J. Lingane and R. L. Pecsok,J. Am. Chem. Soc. February (1949) 425.
G. Charlot, ‘L'analyse Qualitative et les Reactions en Solution,’ Masson et Editeurs Paris (1957).
D. Rai, B. M. Sass and D. A. Moor,Inorg. Chem. 26 (1987) 345.
J. E. Kolakowski and E. Matijevic,J. Chem. Soc. Faraday Trans. I 75 (1979) 65.
H. Cohen, A. Soffer and Y. Oren,J. Coll. Interf. Sci. 120 (1987) 272.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Golub, D., Oren, Y. Removal of chromium from aqueous solutions by treatment with porous carbon electrodes: Electrochemical principles. J Appl Electrochem 19, 311–316 (1989). https://doi.org/10.1007/BF01015228
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01015228