Abstract
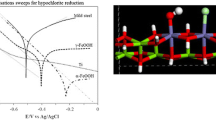
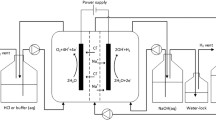
On-site electrolysis of a weak alkaline solution of NaCl has been applied to an increasing extent for disinfection. To optimize the electrolytic cell and the electrolysis conditions, the current efficiency for hypochlorite, chlorate and oxygen formation at a commercial RuO2/TiO2 anode were determined under various conditions. It was found that for solution with low NaCl concentrations, (lower than 200 mol m−3), and at 298 K, solution flow velocity of 0.075 ms−1 and high current density, (higher than 2 kA m−2), hypochlorite formation is determined by mass transfer of chloride. The formation of chlorate in weakly alkaline media at a chlorine and oxygen-evolving anode is ascribed to two reactions, namely, the direct oxidation of chloride to chlorate and the conversion of hypochlorite. This is suggested to split the well-known electrochemical Foerster reaction into a chemical reaction for the conversion of hypochlorite in chlorate and the electrochemical oxidation reaction of water. It is proposed that in an acidic reaction layer at the anode the mechanism of chlorate formation may be given by the following:
Similar content being viewed by others
Abbreviations
- A c :
-
electrode surface area (m2)
- c i :
-
concentration of speciesi (mol m−3)
- c i, 0 :
-
c i att c=0 (mol m−3)
- D i :
-
diffusion constant of speciesi (m2 s−1)
- E :
-
electrode potential versus saturated calomel electrode (V)
- F :
-
Faraday constant (C mol−1)
- i :
-
current (A)
- j :
-
current density (A m−2)
- j d :
-
diffusion-limiting current density (A m−2)
- h i :
-
slope ofn i/c 3 curve (m3 s−1)
- k d, i :
-
diffusion mass-transfer coefficient of speciesi (m s−1)
- k d, f, i :
-
k d, i at forced convection of solution and in the absence of bubble (m s−1) evolution
- N i :
-
quantity of speciesi formed during the electrolysis (mol)
- n i :
-
rate of formation for speciesi (mol s−1)
- n 4, S :
-
n 4 determined by diffusion limitation of hypochlorite (mol s−1)
- t :
-
time (s)
- t e :
-
time of electrolysis (s)
- T :
-
absolute temperature (K)
- v 0 :
-
average flow velocity of solution in the anodic compartment of the cell at the level of the leading edge of the electrode (m s−1)
- Φ i :
-
current efficiency for speciesi
- Φ i, o :
-
Φ i att e=0
- av:
-
average
- d:
-
diffusion
- e:
-
electrolysis, electrode
- f:
-
forced convection
- ox:
-
oxygen
References
N. Ibl and H. Vogt, in ‘Comprehensive Treatise of Electrochemistry’, Vol. 2 (edited by J. O'M. Bockris, B. E. Conway, E. Yeager and R. E. White, Plenum Press, New York and London, (1981), p. 167.
D. M. Novak, B. V. Tilak and B. E. Conway, in ‘Modern Aspects of Electrochemistry’ Vol. 14 (edited by J. O'M. Bockris et al.), Plenum Press, New York (1988). pp. 195–318.
P. Hersch, British Patent 707 323 (awarded to the Mond Nickel Co., Ltd.), 14 April 1954.
W. J. Baker, J. F. Combs, T. L. Zinn, A. W. Wotring and R. F. Wall,Ind. Eng. Chem. 51 (1959) 727.
H. Thielemann,Mikrochimica Acta (Wien) (1971) 746.
T. Tang and G. Gordon,Anal. Chem. 52 (1980) 1430.
Oké—Idem, Environ. Sci. Technol. 18 (1984) 212.
D. IJspeerd, W. H. Willink and H. J. Henning,Fresenius Z. Anal. Chemie 288 (1977) 357.
H. Rilbe, in ‘Electrophoretic Techniques’, (edited by C. F. Simpson and M. Whittaker), Academic Press, London (1983) p. 1.
F. M. Everaerts and T. P. E. M. Verheggen, in ‘Electrophoretic Techniques’, (edited by C. F. Simpson and M. Whittaker), Academic Press, London (1983) p. 1.
L. J. J. Janssen,J. Appl. Electrochem. 17 (1987) 1177.
K. J. Vetter, ‘Elektrochmische Kinetik’, Springer Verlag, Berlin (1961), p. 153.
F. Foerster, ‘Elektrochemie wässriger Lösungen’, 4th ed., G. Bredig Barth, Leipzig (1923) p. 643.
L. R. Czarnetzki, Thesis, Eindhoven, University of Technology (1989).
L. R. Czarnetzki and L. J. J. Janssen, in ‘Modern Chlor-Alkali Technology’, Vol. 4 (edited by N. M. Prout and J. S. Moorhouse), (1990) p. 313.
D. Landolt and N. Ibl,Electrochim. Acta 15 (1970) 1165.
N. Ibl and D. Landolt,J. Electrochem. Soc. 115 (1968) 713.
A. Rius and J. Llopis,An. Fis Quin 41 (1945) 1030.
V. A. Shlyapnikov,Sov. Electrochem. 7 (1971) 1080.
L. R. Czarnetzki and L. J. J. Janssen,Electrochim. Acta 33 (1988) 561.
B. K. Sadananda Rao and V. S. Somanchi,J. Techn (India) 21 (1983) 529.
A. Tasaka and T. Tojo,J. Electrochem. Soc. 132 (1985) 1855.
W. M. Latimer, ‘The oxidation states of the elements and their potentials in aqueous solution’ 2nd edition, Prentice-Hall, Englewood Cliffs, NJ, (1956).
N. Munichandraih and S. Sathyanarayana,J. Appl. Electrochem. 17 (1987) 33.
J. C. Morris,J. Phys. Chem. 70 (1966) 3798.
M. W. Lister,Can. J. Chem. 34 (1956) 465.
—Idem 30 (1952) 879.
J. D'Ans and H. E. Freund,Z. Elektrochemie 61 (1957) 10.
D. V. Kohoulina and L. J. Krishtalik,Elektrokhymiya 7 (1971) 346.
B. V. Tilak, K. Viswanathan and C. G. Radar,J. Electrochem. Soc. 128 (1981) 1228.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Czarnetzki, L.R., Janssen, L.J.J. Formation of hypochlorite, chlorate and oxygen during NaCl electrolysis from alkaline solutions at an RuO2/TiO2 anode. J Appl Electrochem 22, 315–324 (1992). https://doi.org/10.1007/BF01092683
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01092683