Summary
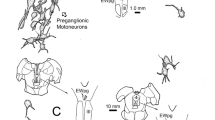
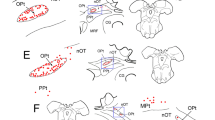
Horseradish peroxidase (HRP) was injected into the anterior eye chamber of rats and mice and frozen sections from both superior cervical and trigeminal ganglia were incubated to demonstrate neurons accumulating the tracer by retrograde axonal transport. Labelled cells were observed only in ganglia ipsilateral to the HRP injection. Within the trigeminal ganglion, peroxidase-containing neurons were restricted to the medial ophthalmic area, whereas labelled cells in the superior cervical ganglion were more widely distributed.
With the use of a new and more sensitive technique for the demonstration of HRP in neurons, it was possible to show retrograde transport also of small amounts of peroxidase injected into the anterior eye chamber. In addition, this technique enabled identification of the central and peripheral processes of neurons in the trigeminal ganglion and the dendrites and axons of sympathetic ganglion cells.
The rate of retrograde HRP transport in rats was calculated to approximately 4–5 mm/h for both sensory and adrenergic nerves, which is consistent with previous estimates for this protein. It differs from the transport rate reported for nerve growth factor (NGF) and macromolecular toxins in sensory and adrenergic nerves of the same species. These rates were, however, obtained with a different method and in a different population of sensory neurons and are, therefore, not directly comparable.
After treatment with 6-hydroxydopamine the number of HRP-labelled cells in the superior cervical ganglion was significantly reduced compared to controls. Cell counts from trigeminal ganglia showed no significant difference between controls and treated animals.
Similar content being viewed by others
References
Arvidson, B. (1977) Retrograde axonal transport of horseradish peroxidase from cornea to trigeminal ganglion.Acta neuropathologica 38, 49–52.
Beatie, J. C. andStilwell, J. R. (1961) Innervation of the eye.Anatomical Record 141, 45–61.
Beaudreau, D. E. andJerge, C. R. (1968) Somatotopic representation in the Gasserian ganglion of tactile peripheral fields in the cat.Archives of Oral Biology 13, 247–56.
Champlain, J., De (1971) Degeneration and regrowth of adrenergic nerve fibers in the rat peripheral tissues after 6-hydroxydopamine.Canadian Journal of Physiology and Pharmacology 49, 345–55.
Darian-Smith, I., Mutton, P. andProctor, R. (1965) Functional organization of tactile cutaneous afferents within the semilunar ganglion and trigeminal spinal tract of the cat.Journal of Neurophysiology 28, 682–94.
Ehinger, B. (1966) Ocular and orbital vegetative nerves.Acta physiologica scandinavica 67, Suppl. 268, 1–35.
Ehinger, B. andFalck, B. (1965) Noradrenaline and cholinesterases in concomitant nerve fibers in rat iris.Life Sciences 4, 2097–100.
Ellison, J. P. andClark, M. C. (1975) Retrograde axonal transport of horseradish peroxidase in peripheral autonomie nerves.Journal of Comparative Neurology 161, 103–14.
Falck, B. (1962) Observations on the possibilities of the cellular localization of monoamine by a fluorescence method.Acta physiologica scandinavica 56, Suppl. 197, 1–25.
Fillenz, M., Gagnon, C., Stoeckel, K. andThoenen, H. (1976) Selective uptake and retrograde axonal transport of dopamine-β-hydroxylase antibodies in peripheral adrenergic neurons.Brain Research 114, 293–303.
Graham, R. C. andKarnovsky, M. J. (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of the mouse kidney. Ultrastructural cytochemistry by a new technique.Journal of Histochemistry and Cytochemistry 14, 291–302.
Hansson, H. A. (1970) Ultrastructure of the surface of the iris in the rat eye.Zeitschrift für Zellforschung und mikroskopische Anatomie 110, 192–203.
Hendry, I. A., Stach, R. andHerrup, K. (1974a) Characteristics of the retrograde axonal transport system for nerve growth factor in the sympathetic nervous system.Brain Research 82, 117–28.
Hendry, I. A., Stoeckel, K., Thoenen, H. andIversen, L. L. (1974b) The retrograde axonal transport of nerve growth factor.Brain Research 68, 103–121.
Huhtala, A., Tervo, T., Huikuri, T. andPalkama, A. (1976) Effects of denervations on the acetylcholinesterase-containing and fluorescent nerves of the rat iris.Acta ophthalmologica 54, 85–98.
Johnson, E. M., Andres, R. Y. andBradshaw, R. A. (1978) Characterization of the retrograde transport of nerve growth factor (NGF) using high specific activity (125I) NGF.Brain Research 150, 319–31.
Jonsson, G. (1971) Effects of 6-hydroxydopamine on the uptake, storage and subcellular distribution of noradrenaline. In6-Hydroxydopamine and Catecholamine Neurons (edited byMalmfors, T. andThoenen, H.), pp. 87–99. Amsterdam, London: North-Holland.
Kaye, G. I., Sibley, R. S. andHoefle, F. B. (1973) Recent studies on the nature and function of the corneal endothelial barrier.Experimental Eye Research 15, 585–613.
Kristensson, K. andOlsson, Y. (1975) Retrograde transport of horseradish peroxidase in transected axons. II. Relations between rate of transfer from the site of injury to the perikaryon and onset of chromatolysis.Journal of Neurcytology 4, 653–61.
Kristensson, K. andOlsson, Y. (1976) Retrograde transport of horseradish peroxidase in transected axons. 3. Entry into injured axons and subsequent localization in perikaryon.Brain Research 115, 201–13.
Kristensson, K., Vahlne, A., Persson, L. A. andLycke, E. (1978) Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation.Journal of the Neurological Sciences 35, 331–40.
Laties, A. andJacobowitz, D. (1966) A comparative study of the autonomic innervation of the eye in monkey, cat and rabbit.Anatomical Record 156, 383–96.
Lavail, J. H. andLavail, M. M. (1974) The retrograde intraaxonal transport of horseradish peroxidase in the chick visual system. A light and electron microscopic study.Journal of Comparative Neurology 157, 303–58.
Leuenberger, P. M. (1973) Lanthanum hydroxide tracer studies on rat corneal endothelium.Experimental Eye Research 15, 85–91.
Mesulam, M.-M. (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents.Journal of Histochemistry and Cytochemistry 26, 106–17.
Raviola, G. (1977) The structural basis of the blood-ocular barriers.Experimental Eye Research 25, (Symposium Suppl.) 27–63.
Rosene, D. L. andMesulam, M. -M. (1978) Fixation variables in horseradish peroxidase neurohistochemistry. I. The effects of fixation time and perfusion procedures upon enzyme activity.Journal of Histochemtstry and Cytochemistry 26, 28–39.
Sakai, A. (1974) Innervation and afferent fiber projection of rat incisor pulp.Bulletin of the Tokyo Medical and Dental University 21, Suppl. 22–4.
Schimert, J. (1936) Untersuchungen über den Ursprung und die Endausbreitung der Nerven der Iris.Zeitschrift für Zellforschung und mikroskopische Anatomie 25, 247–58.
Schwab, M. E. (1977) Ultrastructural localization of a nerve growth factor-horseradish peroxidase (NGF-HRP) coupling product after retrograde axonal transport in adrenergic neurons.Brain Research 130, 190–6.
Stoeckel, K., Paravicini, U. andThoenen, H. (1974) Specificity of the retrograde axonal transport of nerve growth factor.Brain Research 76, 413–21.
Stoeckel, K., Schwab, M. andThoenen, H. (1975a) Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study.Brain Research 89, 1–14.
Stoeckel, K., Schwab, M. andThoenen, H. (1975b) Comparison between the retrograde axonal transport of nerve growth factor and tetanus toxin in motor, sensory and adrenergic neurons.Brain Research 99, 1–16.
Stoeckel, K., Schwab, M. andThoenen, H. (1977) Role of gangliosides in the uptake and retrograde axonal transport of cholera and tetanus toxin as compared to nerve growth factor and wheat germ agglutinin.Brain Research 132, 273–85.
Tervo, T. andPalkama, A. (1976) Adrenergic innervation of the rat corneal epithelium.Investigative Ophthalmology 15, 147–50.
Tønjum, A. M. (1974) Permeability of horseradish peroxidase in the rabbit corneal endothelium.Acta opthalmologica 52, 650–8.
Tranzer, J. P. andRichards, J. G. (1971) Fine structural aspects on the effect of 6-hydroxy-dopamine on peripheral adrenergic neurons. In6-Hydroxy-dopamine and Catecholamine Neurons (edited byMalmfors, T. andThoenen, H.) pp. 15–32. Amsterdam, London: North-Holland.
Tranzer, I. P. andThoenen, H. (1968) An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxy-dopamine.Experientia 24, 155–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arvidson, B. Retrograde transport of horseradish peroxidase in sensory and adrenergic neurons following injection into the anterior eye chamber. J Neurocytol 8, 751–764 (1979). https://doi.org/10.1007/BF01206674
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01206674