Abstract
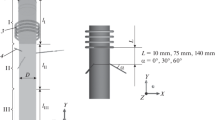
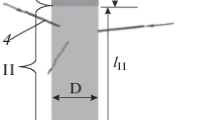
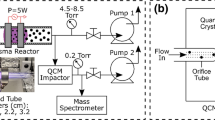
The present paper reports a study of the gas mixing and chemical transformation in an induction plasma reactor under atmospheric pressure, and its dependence on the plasma operating conditions. For this purpose, the thermal dissociation of ammonia into nitrogen and hydrogen was chosen because of the relative simplicity of the reactions involved and its use in a number of studies on plasma synthesis of ultrafine nitride ceramic powders using ammonia as nitriding agent. A hot-wall reactor configuration is investigated in which ammonia is injected radially through multiple orifices into the gases at the exit nozzle of an induction plasma torch. Concentration mapping in the mixing zone was carried out, using a VG-Micromass-PC 300 D quadrupole mass spectrometer, for different plasma power levels, in the range 13–24 kW. A 3-point injection mode is used with the injection ports oriented upstream at 45° to the torch axis. This allows uniform mixing of the injected gas in the plasma jet. A systematic study of the effects of plate power and ammonia and plasma gas flow rates on the mixing and dissociation of NH3 in the reactor is reported. The results are analyzed and discussed from the viewpoint of their use for optimizing the design of induction plasma reactors, to he applied to the vapor-phase synthesis of ultrafine silicon nitride powders.
Similar content being viewed by others
References
G. Soucy, J. W. Jurewicz, and M. I. Boulos,Plasma Chem. Plasma Process. 1, 43 (1994).
G. Soucy, J. W. Jurewicz, and M. I. Boulos,Plasma Chem. Plasma Process. 1, 59 (1994).
N. Kubo, S. Futaki, and K. Shiraishi, “Synthesis of ultrafine Si3N4 powder using the plasma process and powder characterization,” S. Somiya, M. Mitomo, and M. Yoshimura, eds.,Silicon Nitride-1, Ceramic Research and Development in Japan, Vol. 1, Elsevier, New York (1990), p. 93.
T. Yoshida, T. Tami, H. Nishimura, and K. Akashi,J. Appl. Phys. 54, 640 (1983).
T. Hussain and V. J. Ibberson, “Synthesis of ultrafine silicon nitride in an r.f. plasma reactor,” ISPC-7, Eindhoven,2, 692 (1985).
R. Li and W. Gu, “Synthesis of ultrafine and ultrapure silicon nitride powder in an r.f. plasma furnace,” inProduction and Processing of Fine Particles, Proceedings of the International Symposium on the Production and Processing of Fine Particles, A. J. Plumpton, ed., Pergamon Press, New York, (1988) p. 559–568.
J. Szépvölgyi, I. Tóth. and T. Székely,J. Phys. 51, C5–35 (1990).
H. J. Lee, K. Eguchi, and T. Yoshida,J. Am. Ceram. Soc. 73, 3356 (1990).
G. Soucy, “Synthèse de poudres ultrafines de Si3N4 par plasma inductif,” Ph.D. Thesis, Chemical Engineering Department, University of Sherbrooke, Sherbrooke, Québec, Canada (1992).
G. Lantagne, B. Marcos, and B. Cayrol,Comput. Chem. Eng. 12, 589 (1988).
M. W. Chase, C. A. Davies, J. R. Downex, D. J. Frupip, R. A. McDonald, and A. N. Syevrud, 3rd edn.,J. Phys. Chem. Ref. Data 14, Suppl. 1, 1856 (1985).
R. d'Agostino, F. Cramarossa, S. de Benedictis, and G. Ferraro,Plasma Chem. Plasma Process. 1, 19 (1981).
J. E. Nicholas, A. I. Spiers, and N. A. Martin,Plasma Chem. Plasma Process. 6, 39 (1986).
D. Rapakoulias, J. Amouroux, M. P. Bergougnan, and A. Gicquel,Rev. Phys. Appl. 17, 95 (1982).
A. Gicquel, P. Saillard, N. Laidani, and J. Amouroux,Rev. Phys. Appl. 24, 285 (1989).
A. A. Levitskyi, A. A. Ovsyannikov, and L. S. Polak, “Ammonia decomposition in an heated argon flow reactor,” ISPC-3, Limoges paper G.5.9 (1979).
G. Soucy, J. W. Jurewicz, and M. I. Boulos,J. Mater. Sci. 30, 2008 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soucy, G., Jurewicz, J.W. & Boulos, M.I. Parametric study of the decomposition of NH3 for an induction plasma reactor design. Plasma Chem Plasma Process 15, 693–710 (1995). https://doi.org/10.1007/BF01447067
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01447067