Summary
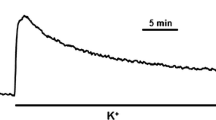
Myometrial strips from oestrogen-primed rat uterus were exposed to various treatments, isometric contraction was measured, and the extent of myosin light chain phosphorylation determined after rapid freezing in liquid nitrogen. Two-dimensional electrophoresis revealed five spots having the same molecular weight as the light chain, with isoelectric points comprised between 5.15 and 4.95. Two of these spots (pI 5.09 and 5.00) were not present in pure uterine myosin, whether prepared from incubated or nonincubated tissue; they do not represent light chain isoforms or electrophoresis artefacts but rather degradation products appearing during the treatment. Two spots (pI 5.15 and 5.06) were identified as the nonphosphorylated and the phosphorylated forms of the light chain. The fifth minor spot (pI 4.95) may represent a diphosphorylated myosin species. Strips incubated in a normal Ca2+-medium (0.8mm) exhibited basal contractions and an incorporation of 0.2 mol phosphate per mol light chain. Removal of Ca2+ resulted in almost complete dephosphorylation, coincident with a total relaxation of the muscle. Exposure of the myometrium to carbachol caused tetanic contractions with an increase to 0.5 mol phosphate per mol light chain. Isoproterenol, a β-adrenergic agonist elevated intracellular cyclic AMP and induced uterine relaxation. Addition of isoproterenol to a resting myometrium caused a slight but significant decrease in phosphorylation; its addition prior to carbachol markedly prevented the increase in myosin phosphorylation normally induced by the cholinergic effector. Forskolin (1 μm) increased intracellular cyclic AMP, caused relaxation and a concomitant decrease in basal myosin phosphorylation. Prostaglandin E2-induced elevation in intracellular cyclic AMP was however accompanied by an increase in contraction together with an increase in light chain phosphorylation. The data imply that light chain phosphorylation-dephosphorylation, regulated by Ca2+-dependent mechanisms, is essential for both uterine contraction and relaxation but question the role of cyclic AMP in exclusively mediating relaxation and myosin dephosphorylation in intact myometrium.
Similar content being viewed by others
References
Adelstein, R. S., Pato, M. D. &Conti, M. A. (1981) The role of phosphorylation in regulating contractile proteins.Adv. Cyclic Nucl. Res. 14, 361–73.
Adelstein, R. S., Sellers, J. R., Conti, M. A., Pato, M. D. & deLanerolle, P. (1982) Regulation of smooth muscle contractile proteins by calmodulin and cyclic AMP.Fedn Proc. Fedn Am. Socs exp. Biol. 41, 2873–8.
Badia, E., Nicolas, J. C., Crastes de Paulet, A., Cavadore, J. C. &Haiech, J. (1985) Myosin light chain phosphorylation during uterine smooth muscle contraction. Effect of hormonal treatments. InCalcium Regulations in Smooth Muscles. Biochemical and Physiological Aspects (edited byMironneau, J.), pp. 333–45. Paris: INSERM.
Bhalla, R. C., Webb, R. C., Singh, D. &Brock, T. (1978) Role of cyclic AMP in rat aortic microsomal phosphorylation and calcium uptake.Am. J. Physiol. 234, H508–14.
Chatterjee, M. &Murphy, R. A. (1983) Calcium-dependent stress maintenance without myosin phosphorylation in skinned smooth muscle.Science, N.Y. 221, 464–6.
Cole, H. A., Griffiths, H. S., Patchell, Y. B. &Perry, S. V. (1985) Two-site phosphorylation of the phosphorylatable light chain (20-kDa light chain) of chicken gizzard myosin.FEBS Lett. 180, 165–9.
Dabrowska, R., Sherry, J. M. F., Aromatorio, D. K. &Hartshorne, D. J. (1978) Modulator protein as a component of the myosin light chain kinase from chicken gizzard.Biochemistry 17, 253–8.
D'Albis, A., Janmot, C. &Bechet, J. J. (1985) Myosin switches in skeletal muscle development of an urodelan amphibian,Pleurodeles waltlii. Comparison with a mammalian, Musmusculus.Biochem. Biophys. Res. Commun. 128, 94–100.
D'Albis, A., Pantaloni, C. &Bechet, J. J. (1979) An electrophoretic study of native myosin isozymes and of their subunit content.Eur. J. Biochem. 99, 261–72.
deLanerolle, P., Nishikawa, M., Yost, D. A. &Adelstein, R. S. (1984) Increased phosphorylation of myosin light chain kinase after an increase in cyclic AMP in intact smooth muscle.Science, N. Y. 223, 1415–7.
deLanerolle, P. &Stull, J. T. (1980) Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle.J. biol. Chem. 255, 9993–10000.
Driska, S. P., Aksoy, M. O. &Murphy, R. A. (1981) Myosin light chain phosphorylation associated with contraction in arterial smooth muscle.Am. J. Physiol. 240, C222–33.
Haeberle, J. R., Hott, J. W. &Hathaway, D. R. (1984) Pseudophosphorylation of the smooth muscle 20 000 dalton myosin light chain. An artifact due to protein modification.Biochim. biophys. Acta 790, 78–86.
Haeberle, J. R., Hott, J. W. &Hathaway, D. R. (1985) Regulation of isometric force and isotonic shortening velocity by phosphorylation of the 20 000 dalton myosin light chain of rat uterine smooth muscle.Pflügers Arch. 403, 215–9.
Harbon, S., Dokhac, L., Janmot, C. &D'Albis, A. (1985) Myosin light chain phosphorylation in intact uterine muscle: effect of contracting and relaxing agents.J. Musc. Res. Cell. Motility 6, 116.
Harbon, S., Dokhac, L. &Vesin, M. F. (1976) Cyclic AMP binding to intracellular receptor proteins in rat myometrium. Effect of epinephrine and prostaglandin E1.Molec. cell. Endocr. 6, 17–34.
Harbon, S., Tanfin-Tougi, Z. &Dokhac, L. (1984) Control of cyclic AMP content of the rat myometrium. β-adrenergic, PGE2 and PGI2 induced stimulation and desensitization. InUterine Contractility (edited byBottari, S., Thomas, J. P., Vokaer, A. andVokaer, R.), pp. 53–69. New York, Paris: Masson.
Harbon, S., Vesin, M. F., Dokhac, L. &Leiber, D. (1978) Cyclic nucleotides in the regulation of rat uterus contractility. InMolecular Biology and Pharmacology of Cyclic Nucleotides (edited byFolco, G. andPaoletti, R.), pp. 279–96. Elsevier, North Holland: Biomedical Press.
Hoh, J. F. Y., McGrath, P. A. &White, R. I. (1976) Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick.Biochem. J. 157, 87–95.
Janis, R. A., Barany, K., Barany, M. &Sarmiento, J. G. (1981) Association between myosin light chain phosphorylation and contraction of rat uterine smooth muscle.Mol. Physiol. 1, 3–11.
Kendrick-Jones, J. &Scholey, J. M. (1981) Myosinlinked regulatory systems.J. Musc. Res. Cell Motility 2, 347–72.
Kerrick, W. G. L., Hoar, P. E. &Cassidy, P. S. (1980) Calcium-activated tension: the role of myosin light chain phosphorylation.Fedn Proc. Fedn Am. Socs exp. Biol. 39, 1558–63.
Laemmli, U. K. &Favre, M. (1973) Maturation of the head of bacteriophage T4. I. DNA packaging events.J. molec. Biol. 80, 575–99.
Ledvora, R. F., Barany, K., Van Der Meulen, D. L., Barron, J. T. &Barany, M. (1983) Stretch-induced phosphorylation of the 20 000 dalton light chain of myosin in arterial smooth muscle.J. biol. Chem. 258, 14080–3.
Marston, S. B. (1983) The regulation of smooth muscle contractile proteins.Progr. Biophys. Mol. Biol. 41, 1–42.
Merkel, L., Meisheri, K. D. &Pfitzer, G. (1984). The variable relation between myosin light-chain phosphorylation and actin-activated ATPase activity in chicken gizzard smooth muscle. Modulation by tropomyosin.Eur. J. Biochem. 138, 429–34.
Miller, J. R., Silver, P. J. &Stull, J. T. (1983) The role of myosin light chain kinase phosphorylation in β-adrenergic relaxation of tracheal smooth muscle.Mol. Pharmac. 24, 235–42.
Mokhtari, A., Dokhac, L., Tanfin, Z. &Harbon, S. (1985) Forskolin modulates cyclic AMP generation in the rat myometrium. Interactions with isoproterenol and prostaglandin E2 and I2.J. Cyclic Nucl. Res. 10, 213–28.
Morrissey, J. H. (1981) Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity.Analyt. Biochem. 117, 307–10.
Nishikawa, M., Hidaka, H. &Adelstein, R. S. (1983) Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid dependent protein kinase.J. biol. Chem. 258, 14069–72
Nishikori, K., Weisbrodt, N. W., Sherwood, O. D. &Sanborn, B. M. (1983) Effects of relaxin on rat uterine myosin light chain kinase activity and myosin light chain phosphorylation.J. biol. Chem. 258, 2468–74.
O'Farrell, P. H. (1975) High resolution two-dimensional electrophoresis of proteins.J. biol. Chem. 250, 4007–21.
Righetti, P. G. &Drysdale, J. W. (1974) Isoelectric focusing in gels.J. Chromatogr. 98, 271–321.
Saida, K. & vanBreemen, C. (1984) Cyclic AMP modulation of adrenoreceptor-mediated arterial smooth muscle contraction.J. gen. Physiol. 84, 307–18.
Sellers, J. R., Chock, P. B. &Adelstein, R. S. (1983) The apparently negatively cooperative phosphorylation of smooth muscle myosin at low ionic strength is related to its filamentous state.J. biol. Chem. 258, 14181–8.
Sobue, K., Moritomo, K., Inui, M., Kando, K. &Kakiuchi, S. (1982) Control of actin interaction of gizzard smooth muscle by calmodulin-and caldesmonlinked flipflop mechanism.Biomed. Res. 3, 188–96.
Sparrow, M. P., Pfitzer, G., Gagelman, M. &Rüegg, J. C. (1984) Effect of calmodulin. Ca2+, and cAMP protein kinase on skinned tracheal smooth muscle.Am. J. Physiol. 246, C308–14.
Vesin, M. F. &Harbon, S. (1974) The effects of epinephrine, prostaglandins and their antagonists on adenosine cyclic 3′, 5′-monophosphate concentration and motility of the rat uterus.Mol. Pharmac. 10, 457–73.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dokhac, L., D'Albis, A., Janmot, C. et al. Myosin light chain phosphorylation in intact rat uterine smooth muscle. Role of calcium and cyclic AMP. J Muscle Res Cell Motil 7, 259–268 (1986). https://doi.org/10.1007/BF01753559
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01753559