Abstract
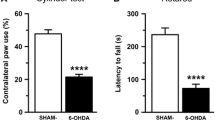
The major symptoms of Parkinson disease (PD) are tremors, hypokinesia, rigidity, and abnormal posture, caused by the degeneration of dopamine (DA) neurons in the substantia nigra (SN) and deficiency of DA in the neostriatal DA terminals. Norepinephrine (NE) and serotonin (5-HT) levels in the neostriatum and tyrosine hydroxylase and melanin pigments in the substantia nigra are also decreased, and brain cholinergic activity is increased. The cause of PD is unknown, but PD is an age-related disorder, suggesting that changes that occur during the aging process may help to precipitate PD. Methylation increases in aging animals. Increased methylation can deplete DA, NE, and 5-HT; increase acetylcholine; and cause hypokinesia and tremors. These effects are similar to changes seen in PD, and interestingly also, they are similar to some of the changes that are associated with the aging process. It is suggested, therefore, that increased methylation may be an inducing factor in parkinsonism. Accordingly, the effects of an increase in methylation in the brain of rats were studied.S-adenosylmethionine (AdoMet), the limiting factor in the methylation process, was injected into the lateral ventricle of rats. Specific behavioral changes that resemble changes seen in PD were investigated. The results showed that AdoMet caused tremors, rigidity, hypokinesia, and depleted DA. The hypokinetic effects of a single dose of AdoMet lasted for about 90 min. AdoMet has a dose-dependent hypokinetic effect. A dose of 9.4 nmol reduced movement time (MT) by 68.9% and increased rest time (RT) by 20.7%, and a dose of 400 mnol reduced MT by 92.4% and increased RT by 27.6%. The normethyl analog of AdoMet,S-adenosylhomocysteine, did not cause hypokinesia or tremors, but it blocked the AdoMet-induced motor effects.l-dopa, the precursor of DA, also blocked the AdoMet-induced motor effects. These data suggest that the methyl group of AdoMet as well as DA depletion are involved in the AdoMet-induced motor effects. A dose of 0.65 μmol of AdoMet depleted DA in the ipsilateral caudate nucleus (CN) or neostriatum by 50.1%, and DA in the contralateral CN was reduced by 9.3%. Double the dose of AdoMet did not increase the depletion of DA on the ipsilateral CN, but DA in the contralateral CN was decreased by 26.3%. Taken together, the results suggest that increased methylation may contribute to the symptoms of PD.
Similar content being viewed by others
References
Alvord E. C., Jr., Forno L. S., Kusske J. A., Kaufman R. J., Rhodes J. S., and Goetowski C. R. (1974) The pathology of parkinsonism: comparison of degeneration in cerebral cortex and brainstem.Adv. Neurol. 5, 175–193.
Baldessarini R. J. and Kopin I. J. (1966)S-adenosylmethionine in brain and other tissues.J. Neurochem. 13, 769–777.
Barbeau A., Tetreault L., Morazain L., and Oliva L. (1965) Pharmacology of 3,4-dimethoxyphenylamine.Can. Med. Assoc. J. 92, 347.
Barbeau A. (1968) Dopamine and dopamine metabolites in Parkinson’s disease— a review.Proc. Aust. Assoc. Neurol. 5, 95–100.
Blusztajn J. K., Zeisel S. H., and Wurtman R. J. (1979) Synthesis of lecithin (phosphatidylcholine) frm phosphatidylethanolamine in bovine brain.Brain Res. 179, 319–327.
Blusztajn J. K. and Wurtman R. J. (1983) Choline and cholinergic neurons.Science 221, 614–620.
Charlton C. G. and Way E. L. (1978) Tremor induced byS-adenosyl-L-methionine: possible relation tol-dopa effects.J. Pharma. Pharmacol. 30, 819–820.
Charlton C. G. and Crowell B., Jr. (1990) Relationship between excessS-adenosylmethionine (SAM-dependent methylation) and Parkinson’s disease.Neurosci. Abstr. 16(1), 810.
Charlton C. G. and Crowell B. Jr. (1992) Parkinson’s disease-like effects ofS-adenosyl-l-methionine: effects ofl-dopa.Pharm. Biochem. Behav. 43, 423–431.
Clarke S. (1992) Protein isoprenylation and methylation at carboxyl-terminal cysteine residues.Annu. Rev. Biochem. 61, 355–386.
Collins M. A., Neafsey E. J., Matsubara K., Cobuzzi R. J., Jr., and Rollema H. (1992) Indole-N-methylated beta-carbolinium ions as potential brain-bioactivated neurotoxins.Brain Res. 570, 154–160.
Coyle J. T. and Henry D. (1973) Catecholamines in fetal and newborn rat brain.J. Neurochem. 21, 61–67.
Crowell B., Jr., Benson R., Shockley D., and Charlton C. G. (1993)S-adenosyl-l-methionine decreases motor activity in rat: similarity to Parkinson’s disease-like symptoms.Behav. Neural Biol. 59, 186–193.
De Olivera Filgueiras O. M., Van Den Basselaar A. H. H. P., and Van Den Bosch H. (1979) Localization of lysophosphatidylcholine in bovine chromaffin granules.Biochim. Biophys. Acta 558, 73–84.
Diliberto E. J., Jr., and Axelrod J. (1976) Regional and subcellular distribution of protein carboxymethylase in brain and other tissues.J. Neurosci. 26, 1159–1165.
Diliberto E. J., Jr., Viveros O. H., and Axelrod J. (1976) Subcellular distribution of protein carboxymethylase and its endogenous substrates in the adrenal medulla: possible role in excitation-secretion coupling.Proc. Natl. Acad. Sci. USA 73, 4050–4054.
Eadie M. J. (1963) The pathology of certain medullary nuclei in parkinsonism.Brain 86, 781–790.
Ernst A. M. (1962) Phenomena of the hypokinetic rigid type caused by O-methylation of dopamine in the para-position.Nature (Lond) 193, 178, 179.
Feuerstein C., Tauche M., Serre F., Gavend M., Pellat J., and Perret J. (1977) Does O-methylation play a role in levo-dopa-induced dyskinesias?Acta Neurol. Scand. 56, 79–82.
Forno L. S. and Norvill R. L. (1976) Ultrastructure of Lewy bodies in the stellate ganglion.Acta Neuropathol. 34, 183–197.
Gagnon C., Viveros O. H., Diliberto E. J., and Axelrod J. (1978) Enzymatic methylation of carboxyl groups of chromaffin granule membrane proteins.J. Biol. Chem. 253, 3778–3781.
Ganong W. F. (1991)Review of Medical Physiology. Appleton and Lange, San Francisco, CA.
Gharib A., Sarda N., Chabannes B., Cronenberger L., and Pacheco H. (1982) The regional concentrations ofS-adenosyl-l-methionine,S-adenosyl-l-homocysteine and adenosine in rat brain.J. Neurochem. 38, 810–815.
Goodman L. S. and Gilman A. (1981)The Pharmacological Basis of Therapeutics. Macmillan, New York.
Hardie R. J., Lees A. J., and Stern G. M. (1986) Pharmacokinetics of levo-dopa and motor fluctuations.Adv. Neurol. 45, 487–492.
Hirata F. and Axelrod J. (1978) Enzymatic methylation of phosphatidylethanolamine increases erythrocyte membrane fluidity.Nature 275, 219, 220.
Jager D. H. and Bethlem J. (1960) The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans.J. Neurol. Neurosurg. Psychiatr. 6, 283–290.
Jager W. A. den (1969) Sphingomyelin in Lewy inclusion bodies in Parkinson’s disease.Arch. Neurol. (Chicago) 21, 615–619.
Jenner P. J. and Marsden C. D. (1988) MPTP-induced parkinsonism as an experimental model of Parkinson’s disease. In:Parkinson’s Disease and Movement Disorders (Jankovic J. and Tolosa E., eds.), pp. 37–48, Urban & Schwarzenberg, Baltimore-Munich.
Knoll J. (1988) The striatal dopamine dependency of life span in male rats, longivity study with (−) deprenyl.Mech. Aging Dev. 46, 237–262.
Langston J. W. and Forno L. S. (1978) The hypothalamus in Parkinson disease.Ann. Neurol. 3, 129–133.
Maksem J., Jacobson N., and Neiderhiser D. H. (1984) Lysophosphatidylcholine-induced gastric injury and ulceration in the guinea pig.Am. J. Pathol. 115, 288–295.
Mays L. I., Borek E., and Finch C. E. (1973) Glycine N-methyltransferase is a regulatory enzyme which increases in aging animals.Nature 243, 411–413.
Mena M. A., Murados V., Brazen E., Reiriz J., and De Yebenes J. G. (1977) Pharmacokinetics ofl-dopa in patients with Parkinson’s disease.Adv. Neurol. 45, 481–486.
Muenter M. D., Sharpless N. S., and Tyce G. M. (1972) Plasma 3-O-methyldopa inl-dopa therapy of Parkinson’s disease.Mayo Clin. Proc. 47, 389–395.
Ohama E. and Ikuta F. (1976) Parkinson’s disease: distribution of Lewy bodies and monoamine neuron system.Acta Neuropathol. (Berl) 34, 311–319.
Phillips M. R., Pillinger M. H., Strand R., Volker C., Rosenfeld M. G., Weissman G., and Stock J. B. (1993) Carboxymethylation of Ras-related proteins during signal transduction in neurophils.Science 259, 977–980.
Rajput A. H. and Rozdisky B. (1970) Dysautonomia in parkinsonism: a clinico-pathological study.J. Neurol. Neurosurg. Psychiatr. 39, 1092–1100.
Schultz W. (1988) MPTP-induced parkinsonism in monkeys: mechanisms of action, selectivity and pathophysiology.Gen. Pharmacol. 19, 153–161.
Selby G. (1968) Cerebral atrophy in parkinsonismJ. Neurol. Sci. 6, 517–559.
Sellinger O. Z., Kramer C. M., Conger A., and Duboff G. S. (1988) The carboxylmethylation of cerebral membrane-bound proteins increases with age.Mech. Aging Dev. 43, 161–173.
Sharman D. F. (1976) The effect of drugs on dopamine in the striatum. In:Third Symposium on Parkinson’s Disease (Gillingham F. J. and Donaldson I. M. L., eds.), pp. 24–32, E and L Livingston, London.
Slomiany B. L., Jerzy-Glass G. B., Kojima K., Banas-Gruszka Z., and Slomiany A. (1981) Effect of lysolecithin on the constitutents of gastric mucus. In:International Symposium on Mucus in Health and Disease (Chantler N., Elder J. B., and Elstein M., eds.), pp. 163–174, Plenum, New York.
Stramentinoli G., Gualano M., Catto E., and Algeri S. (1977) Tissue levels ofS-adenosylmethionine in aging rats.J. Gerontol. 32, 392–394.
Taufek H. R. and Bone A. H. (1984) Influence of exogenousl-3,4-hydroxyphenylalanine (l-dopa) on methionine andS-adenosylmethionine concentrations in the brain and other tissues.Biochem. Soc. Trans. 8, 62–63.
Tuomisto L. (1977) Ontogenesis and regional distribution of histamine and histamine-N-methyltransferase in the guinea pig brain.J. Neurochem. 28, 271–276.
Vanderhaegen J. J., Poirier O., and Steronon J. E. (1970) Pathological findings in idiopathic orthostatic hypotension.Arch. Neurol. 22, 207–214.
Volpe J. J. and Laster L. (1970) Trans-sulphuration in primate brain: regional distribution of methionine-activating enzyme in the brain of the Rhesus monkey at various stages of development.J. Neurochem. 17, 413–424.
Weltzien H. U. (1979) Cytolytic and membrane perturbing properties of lysophosphatidylcholine.Biochim. Biophys. Acta 559, 259–287.
Wurtman R. J., Rose C. M., Matthysse S., Stephenson J., and Baldessarini R. J. (1970)l-dihydroxyphenylalanine: effect onS-adenosylmethionine in brain.Science 169, 395–397.
Yahr M. D. and Bering E. A. (1968)Parkinson’s Disease: Present Status and Research Trends. Columbia University Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Charlton, C.G., Crowell, B. Striatal dopamine depletion, tremors, and hypokinesia following the intracranial injection ofS-adenosylmethionine. Molecular and Chemical Neuropathology 26, 269–284 (1995). https://doi.org/10.1007/BF02815143
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02815143