Abstract
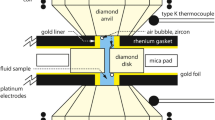
The electrical conductivity of H2O in solid and liquid phases has been measured at 0.21–4.18 GPa and 20–350°C. The results indicate: (I) different phases of H2O in solid have different relations between electrical conductivity and temperature and pressure. The conductivity changes continuously with temperature, but discontinuously with the pressure between 2.11 and 2.58 GPa, which corresponds to the transforming pressure between ice (VI) and ice (VII);(II) the amductivity of H2O in liquid all increases with temperature and pressure, but there are discontinuities at pressures between 0.57 and 0.9 GPa, and between 2.11 and 2.58 GPa, which are also consistent with the polyrnorph of ice (ice (V), ice (VI) and ice (VII)). This reflects that H2O in liquid at different pressures has quite different properties of electron chemistry. It is probably the important reason that causes the layers with high electrical conductivity and low velocity in the earth’s ceust and upper mantle.
Similar content being viewed by others
References
Watson, G. W., Wall, A., Parker, S. C., A molecular dynamics simulation of the effect of high pressure on fastion mnduction in a MgSiO3-pedite analogue-KCAF3.Physics of the Earth and Planetary Interiurs, 1995. 89(1–2): 137.
Hyndman, R. D., Vanyan, L. I,., Marquis, G.et al., The origin of electrically conductive lower continental crust: saline water or graghite?Phys. Earth Planet. Int., 1993, 81, 325.
Holzapfel, W. B., Effect of pressure and temperature on the conductivity and ionic dissociation of water up to 10 kbar and 1000°C.J. Chem. Phys., 1969, 50, 4424.
Manhall, W. L., Franck, E. U., Ion product of water substance at 0–1000°C. 1–10 000 bar, new international formulation and its background,Phys. Chem. Ref. Data, 1981, 10, 295.
Tanger, J. C., Pitzer, K: S., Calculation of the ionization constant of H2O to 2 273 K and 500 MPa,AICHE J., 1989, 35: 1631.
Eben, A., Franck, E. U., High pressure electrolyte conductivity of the homogeneous, fluid water-sodium hydroxide system to 400°C and 300 bar,Berichte der Bunsengesellschuft fiir physikulische Chcmie, 1995, 99(9): 1091.
Ho, P. C., Palmer, D. A., Mesmer, R. E., Electrical conductivity measurements of aqueous sodium chloride solutions to 600°C and 300 MPa,Journal of Solution Chemistry, 1994, 23 (9): 997.
Holland, T., Powell, R., Compensated-Redlich-Kwong (CORK) equation for volumes and fugacities of CO2 and H2O in the range 1 bar to 50 kbar and 100–1 600°C,Gontrib. Mineral. Petrol., 1991, 109: 265.
Liu Lingun, Willham, A. B.,Ehcnts, Ozides, Silicates: High-Pressure Phases with Implications for the Farth’s Interior, New York and Clarendon, Oxford: Oxford University Press, 1986, 92.
Quist, A. S., Manhall, W. L., Electrical conductances of aqueous sodium chloride solutions from 0 to 800°C and at pressures of 4 000 bars,J. Phys. Chem., 1968, 72(2): 684.
Wang Minghao, Qin Shunting, Qi Fengwuet al., Physical Propcrly of Rods and its Application in Oil Ezploration (in Chinese), Beijing: Geological Publishing House, 1994, 129.
Wu Qibin, Zhang Zhaoyuan, Global geoxience transects project, inStudy of Crust and Upper Mantle, (eds. Xiang Renjieet al.) (in Chinese), Beijing: Seiidogical Press, 1991, 25–39.
Zheng Haifei. Xie Hongsen, Xu Youshenget al., Experimental study of the influence of hydrous minerals on the melting behaviour of rucks at high temperatures and pressures.Acta Grvlogica Sinica (in Chinese), 1995, 9(2): 157.
Author information
Authors and Affiliations
About this article
Cite this article
Zheng, H., Xie, H., Xu, Y. et al. The electrical conductivity of H2O at 0.21– 4.18 GPa and 20–350°C. Chin.Sci.Bull. 42, 969–976 (1997). https://doi.org/10.1007/BF02882610
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02882610