Abstract
Intention, Goal, Scope, Background
Cyanobacteria have the natural ability to degrade moderate amounts of organic pollutants. However, when pollutant concentration exceeds the level of tolerance, bleaching of the cells and death occur within 24 hours. Under stress conditions, cyanobacterial response includes the short-term adaptation of the photosynthetic apparatus to light quality, named state transitions. Moreover, prolonged stresses produce changes in the functional organization of phycobilisomes and in the core-complexes of both photosystems, which can result in large changes in the PS II fluorescence yield. The localization of ferredoxin-NADP+ reductase (FNR) at the ends of some peripheral rods of the cyanobacterial phycobilisomes, makes this protein a useful marker to check phycobilisome integrity.
Objective
The goal of this work is to improve the knowledge of the mechanism of action of a very potent pesticide, lindane (γ-hexaclorociclohexane), in the cyanobacteriumAnabaena sp., which can be considered a potential candidate for bioremediation of pesticides. We have studied the effect of lindane on the photosynthetic apparatus ofAnabaena using fluorescence induction studies. As ferredoxin-NADP+ reductase plays a key role in the response to oxidative stress in several systems, changes in synthesis, degradation and activity of FNR were analyzed. Immunolocalization of this enzyme was used as a marker of phycobilisome integrity. The knowledge of the changes caused by lindane in the photosynthetic apparatus is essential for rational further design of genetically-modified cyanobacteria with improved biorremediation abilities.
Methods
Polyphasic chlorophylla fluorescence rise measurements (OJIP) have been used to evaluate the vitality and stress adaptation of the nitrogen-fixing cyanobacteriumAnabaena PCC 7119 in the presence of increasing concentrations of lindane. Effects of the pesticide on the ultrastructure have been investigated by electron microscopy, and FNR has been used as a marker of phycobilisome integrity.
Results and Discussion
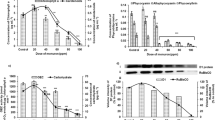
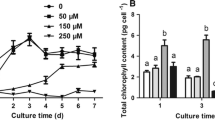
Cultures ofAnabaena sp. treated with moderate amounts of lindane showed a decrease in growth rate followed by a recovery after 72 hours of pesticide treatment. Concentrations of lindane below 5 ppm increased the photosynthetic performance and activity of the cells. Higher amounts of pesticide caused a decrease in these activities which seems to be due to a non-competitive inhibition of PS II. Active PS II units are converted into non-QA reducing, so called heat sink centers. Specific activity and amount of FNR in lindane-treated cells were similar to the values measured in control cultures. Release of FNR from the thylakoid after 48 hours of exposure to 5 ppm of lindane towards the cytoplasm was detected by immunogold labeling and electron microscopy.
Conclusions
From these results, we conclude that the photosynthetic performance and activity of the cells are slightly increased in the presence of lindane up to 5 ppm. Moreover, in those conditions, iindane did not produce significant changes in the synthesis, degradation or activity of FNR. The high capability ofAnabaena to tolerate lindane makes this cyanobacterium a good candidate for phytoremediation of polluted areas.
Recommendation and Outlook
The results of this study show that cultures ofAnabaena PCC 7119 tolerate lindane up to 5 ppm, without significant changes in the photosynthetic vitality index of the cells. However, a slight increase in phycobiliprotein synthesis is observed, which is related to total protein content. This change might be due to degradation of proteins less stable than phycobiliproteins. An identification of the proteins with altered expression pattern in the presence of the pesticide remains the subject of further work and will provide valuable information for the preparation of strains which are highly tolerant to lindane.
Similar content being viewed by others
Abbreviations
- CS:
-
cross section of the sample
- DI:
-
dissipated flux
- ET:
-
energy flux for electron transport
- Fo, FM :
-
initial and maximum Chla fluorescence
- J, I:
-
intermediate steps of Chla fluorescence rise between Fo and peak (P)
- kN :
-
non-photochemical, de-excitation rate constant
- kp :
-
photochemical de-excitation rate constant
- ϕEO :
-
probability that an absorbed photon will move an electron into the electron transport chain
- ϕPo. :
-
maximum quantum yield of primary photochemistry
- ψ0 :
-
efficiency with which a trapped exciton can move an electron into the electron transport chain
- QA :
-
primary bound plastoquinone
- QB :
-
secondary bound plastoquinone
- RC:
-
reaction center
- TR:
-
energy flux for trapping
References
Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK (1999): Biochemical effects of some pesticides on lipid peroxidation and freeradical scavengers. Toxicol Lett 107, 33–47
Brack W, Frank H (1998): Chlorophyll a fluorescence: a tool for the investigation of toxic effects in the photosynthetic apparatus. Ecotoxicol Environ Safety 40, 34–41
Bradford MM (1976): A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteinsdye binding. Anal Biochem 72, 248–254
Bryant DA (1994): The Molecular Biology of Cyanobacteria (Bryant DA, Ed.), Kluwer Academic Publishers, Dordrecht, The Netherlands, 908 pp.
Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G (1998): Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62, 667–683
Das B, Singh PK (1978): Pesticide (hexachlorocyclohexane) inhibition of growth and nitrogen fixation in blue-green algaeAnabaenopsis raciborskii andAnabaena aphanizomenoides. Z allgem Mikrobiol 18, 161–167
DaSilva EJ, Henriksson LE, Henriksson E (1975): Effect of pesticides on blue-green algae and nitrogen-fixation. Arch Environ Contam Toxicol 3, 193–204
Fay P (1992): Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 56, 340–373
Fillat MF, Edmondson DE, Gomez-Moreno C (1991): Light-dependent de-activation/re-activation ofAnabaena variabilis ferredoxin: NADP+ reductase. Biochem J 274, 781–786
Fillat MF, Flores E, Gomez-Moreno C (1993): Homology of the N-terminal domain of thepetH gene product fromAnabaena sp. PCC 7119 to the CpcD phycobilisome linker polypeptide. Plant Mol Biol 22, 725–729
Glazer AN (1976): Phycocyanins: Structure and function. Photochem Photobiol Rev 1, 71–115
Glazer AN (1989): Light guides. Directional energy transfer in a photosynthetic antenna. J Biol Chem 264, 1–4
Havaux M, Strasser RJ, Greppin HA (1991): A theoretical and experimental analysis of the qp and qn coefficients of chlorophyll fluorescence quenching and their relation to photochemical and non photochemical events. Photosynth Res 27, 41–55
Krause, GH, Weis E (1991): Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42, 313–349
Krüger GHJ, Tsimilli-Michael M, Strasser RJ (1997): Light stress provokes plastic and elastic modifications in structure and function of photosystem II in camelia leaves. Physiol Plant 101, 265–277
Kuritz T, Wolk CP (1995): Use of filamentous cyanobacteria for biodegradation of organic pollutants. Appl Environ Microbiol 61, 234–238
Kuritz T, Bocanera LV, Rivera NS (1997): Dechlorination of lindane by the cyanobacteriumAnabaena sp. strain PCC7120 depends on the function of thenir operon. J Bacteriol 179, 3368–3370
Liochev SI, Hausladen A, Beyer Jr WF, Fridovich I (1994): NADPH: ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of thesoxRS regulon. Proc Natl Acad Sci USA 91, 1328–1331
Mackinney G (1941): Absortion of light by chlorophyll solutions. J Biol Chem 140, 315–322
Martinez-Julvez M, Hurley JK, Tollin G, Gómez-Moreno C, Fillat MF (1996): Overexpression inE. coli of the complete petH gene product fromAnabaena: purification and properties of a 49 kDa ferredoxin- NADP+ reductase. Biochim Biophys Acta 1297, 200–206
Palatnik JF, Valle EM, Carrillo N (1997): Oxidative stress causes ferredoxin-NADP+ reductase solubilization from the thylakoid membranes in methyl viologen-treated plants. Plant Physiol 115, 1721–1727
Razquin P, Fillat MF, Gómez-Moreno C, Peleato ML (1995): The 36 kDa form of ferredoxin-NADP+reductase fromAnabaena co-purifies with phycobiliproteins. Bioelectrochem Bioen 38, 57–61
Rippka R, Deruelles j, Waterbury JB, Herdman M, Stanier RY (1979): Genetic assignments, strains histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 11, 1–61
Sancho J, Peleato ML, Gomez-Moreno C, F.dmondson DE (1988): Purification and properties of ferredoxin-NADP+ oxidoreductase from the nitrogen-fixing cyanobacteriaAnabaena variabilis. Arch Biochem Biophys 260, 200–207
Sidler WA (1994): Phycobilisome and phycobiliprotein structures. In: Bryant DA (Ed): The Molecular Biology of Cyanobacteria,. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 139–216
Smith D, Bendall DS, Howe CJ (1992): Occurrence of a Photosystem II polypeptide in non-photosynthetic membranes of cyanobacteria. Mol Microbiol 6, 1821–1827
Srivastava A, Guissé B, Greppin H, Strasser RJ (1997): Regulation of antenna structure and electron transport in photosystem II ofPisum sativum under elevated temperature probed by the fast polyphasic chlorophylla fluorescence transient: OKJIP. Biochim Biophys Acta 1320, 95–106
Strasser BJ (1997): Donor side capacity of photosystem II probed by chlorophylla fluorescence transients. Photosynth Res 52, 147–155
Strasser RJ, Srivastava A, Govindjee (1995): Polyphasic chlorophylla fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61, 32–42
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000): The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanthy P (Eds): Probing Photosynthesis, Mechanism, Regulation and Adaptation, Taylor & Francis, London, U.K, pp. 445–183
Strasser RJ, Tsimilli-Michael M (2001): Stress in plants, from daily rhythm to global changes, detected and quantified by the JIP-test. Chimie Nouvelle (SRC) 75, 3321–3326
Suresh Babu G, Hans R, Singh J, Viswanathan PN, Joshi PC (2001): Effect of lindane on the growth and metabolic activities of cyanobacteria. Ecotoxicol Environ Safety 48, 219–221
van Thor JJ, Gruters OWM, Matthijs HCP, Hellingwerf KJ (1999): Localization and function of ferredoxin: NADP+ reductase bound to the phycobilisomes of Synechocystis. EMBO J 18, 4128–4136
Wang RT, Myers J (1973): Energy transfer between photosynthetic units analyzed by flash oxygen yield vs. flash intensity. Photochem Photobiol 17, 321–332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bueno, M., Fillat, M.F., Strasser, R.J. et al. Effects of lindane on the photosynthetic apparatus of the cyanobacteriumanabaena . Environ Sci & Pollut Res 11, 98–106 (2004). https://doi.org/10.1007/BF02979709
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02979709