Abstract
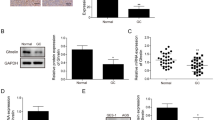
Ghrelin, a natural GH secretagog (GHS) acylated peptide, and cortistatin (CST), a natural SRIF-like peptide, interfere with neoplastic growth in different cancers. We tested forty-one lung carcinomas and the H345 small cell lung carcinoma (SCLC) cell line by RT-PCR to investigate the presence of ghrelin and CST and related receptors, including type 1a GHS receptor (GHS-R1a), all SRIF-receptor subtypes (sst 1–5) and MRGX2. Moreover, the presence of ghrelin and CST peptides was studied in both tumors and H345 cells. Ghrelin and CST mRNA were present in the majority of tested tumors, but ghrelin and CST proteins were revealed only in tumors with a neuroendocrine phenotype. All the receptors mRNA had a heterogeneous expression without correlation between ghrelin (or CST) and their receptor distribution. All the transcripts, but not GHS-R1a, were expressed in H345 cells. However, ghrelin and desacyl ghrelin induced in vitro a dose-dependent inhibition on the H345 cell proliferation and increased apoptosis. Conversely, neither CST nor SRIF affected H345 cell growth, despite the presence of their specific receptors. The anti-proliferative and the pro-apoptotic effects of ghrelin were consistent with binding experiments on H345 cell, where either acylated or des-acylated ghrelin recognized a common binding site. In conclusion, the present study indicates that: a) ghrelin and CST mRNAs are expressed in lung cancers, although some neuroendocrine tumors contain detectable amounts of the peptides; b) GHSR-1a mRNA is present exclusively in neuroen-docrine tumors, whereas MRGX2 mRNA (but not peptide) is expressed in all histological types; c) both ghrelin forms inhibit H345 cell proliferation, both directly and enhancing apoptosis, despite the absence of GHS-R1a, whereas CST and its receptors do not interfere with cell growth.
Similar content being viewed by others
References
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone acylated peptide from stomach. Nature 1999, 402: 656–66.
Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 2001, 12: 118–22.
van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004, 25: 426–57.
Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin-ahormone with multiple functions. Front Neuroen-docrinol 2004, 25: 27–68.
Arvat E, Maccario M, di Vito L, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnaturalpeptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 2001, 86: 1169–74.
Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141: 4325–8.
Muccioli G, Tschop M, Papotti M, Deghenghi R, Heiman M, Ghigo E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol 2002, 440: 235–54.
Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem 2000, 275: 21995–2000.
Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 2000, 279: 909–13.
Torsello A, Ghe’ C, Bresciani E, et al. Short ghrelin peptides neither displace ghrelin binding in vitro nor stimulate GH release in vivo. Endocrinology 2002, 143: 1968–71.
Broglio F, Benso A, Gottero C, et al. Non-acylated ghrelin does not possess the pituitaric and pancreatic endocrine activity of acylated ghrelin in humans. J Endocrinol Invest 2003, 26: 192–6.
Gualillo O, Caminos J, Blanco M, et al. Ghrelin, a novel placental-derived hormone. Endocrinology 2001, 142: 788–94.
Volante M, Allia E, Gugliotta P, et al. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab 2002, 87: 1300–8.
Korbonits M, Bustin SA, Kojima M, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 2001, 86: 881–7.
Kim K, Arai K, Sanno N, Osamura RY, Teramoto A, Shibasaki T. Ghrelin and growth hormone (GH) secretagogue receptor (GHSR) mRNA expression in human pituitary adenomas. Clin Endocrinol (Oxf) 2001, 54: 759–68.
Papotti M, Cassoni P, Volante M, Deghenghi R, Muccioli G, Ghigo E. Ghrelin-producing endocrine tumors of the stomach and intestine. J Clin Endocrinol Metab 2001, 86: 5052–9.
Yeh AH, Jeffery PL, Duncan RP, Heritngton AC, Chopin LK. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogenactivated protein kinase in prostate cancer. Clin Cancer Res 2005, 11: 8295–303.
Volante M, Allia E, Fulcheri E, et al. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am J Pathol 2003, 162: 645–54.
Howard AD, Feighner SC, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273: 974–7.
McKee KK, Palyha OC, Feighner SD, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol 1997, 11: 415–23.
Barzon L, Pacenti M, Masi G, Stefani AL, Fincati K, Palu G. Loss of growth hormone secretagogue receptor 1a and overexpression of type 1b receptor transcripts in human adrenocortical tumors. Oncology 2005, 68: 414–21.
Cassoni P, Papotti M, Ghe C, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 2001, 86: 1738–45.
Cassoni P, Ghe C, Marrocco T, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur J Endocrinol 2004, 150: 173–84.
Belloni AS, Macchi C, Rebuffat P, et al. Effect of ghrelin on the apoptotic deletion rate of different types of cells cultured in vitro. Int J Mol Med 2004, 14: 165–7.
Ghe C, Cassoni P, Catapano F, et al. The antiproliferative effect of synthetic peptidyl GH secretagoguesin human CALU-1 lung carcinoma cells. Endocrinology 2002, 143: 484–91.
Spier AD, de Lecea L. Cortistatin: a member of the somatostatin neuropeptide family with distinct physiological functions. Brain Res Brain Res Rev 2000, 33: 228–41.
Broglio F, Arvat E, Benso A, et al. Endocrine activities of cortistatin-14 and its interaction with GHRH and ghrelin in humans. J Clin Endocrinol Metab 2002, 87: 3783–90.
Allia E, Tarabra E, Volante M, et al. Expression of cortistatin and MrgX2, a specific cortistatin receptor, in human neuroendocrine tissues and related tumours. J Pathol 2005, 207: 336–45.
Cassoni P, Muccioli G, Marrocco T, et al. Cortistatin-14 inhibits cell proliferation of human thyroid carcinoma cell lines of both follicular and parafollicular origin. J Endocrinol Invest 2002, 25: 362–8.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology and Genetics. Tumors of lung pleura, thymus and heart. In WHO classification of tumors. Lyon: IARC Press (ed). 2004.
Volante M, Fulcheri E, Allia E, Cerrato M, Pucci A, Papotti M. Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem 2002, 50: 1013–21.
Muccioli G, Papotti M, Locatelli V, Ghigo E, Deghenghi R. Binding of 1251-labeled ghrelin to membranes from human hypothalamus and pituitary gland. J Endocrinol Invest 2001, 24: RC7–9.
Gerling N, Culmsee C, Klumpp S, Krieglstein J. The tyrosine phosphatase inhibitor orthovanadate mimics NGF-induced neuroprotective signaling in rat hippocampal neurons. Neurochem Int 2004, 44: 505–20.
Bednarek MA, Feighner SD, Pong SS, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 2000, 43: 4370–6.
Kanamoto N, Akamizu T, Hosoda H, et al. Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J Clin Endocrinol Metab 2001, 86: 4984–90.
Jeffery PL, Herington AC, Chopin LK. Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J Endocrinol 2002, 172: R7–11.
Gaytan F, Barreiro ML, Caminos JE, et al. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab 2004, 89: 400–9.
Arnaldi G, Mancini T, Kola B, et al. Cyclical Cushing’s syndrome in a patient with a bronchial neuroendocrine tumor (typical carcinoid) expressing ghrelin and growth hormone secretagogue receptors. J Clin Endocrinol Metab 2003, 88: 5834–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassoni, P., Allia, E., Marrocco, T. et al. Ghrelin and cortistatin in lung cancer: Expression of peptides and related receptors in human primary tumors and in vitro effect on the H345 small cell carcinoma cell line. J Endocrinol Invest 29, 781–790 (2006). https://doi.org/10.1007/BF03347371
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347371