Abstract
Objective
This cross-sectional study assessed the potential contribution of gender, body fat distribution, and their interactions to some inflammatory marker concentrations [C-reactive protein (CRP), complement factor 3 (C3), and ceruloplasmin (Cp)] in young adults.
Methods
Measurements included body composition, lifestyle features, blood biochemical and selected inflammatory markers on 317 healthy subjects [122 males/195 females; 22 ± 3 years; 22.1 ± 2.8 kg/m2 (mean ± SD)].
Results
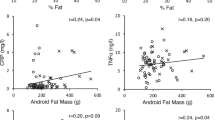
Women had significantly higher CRP and Cp concentrations than men. No gender difference was noted in C3 concentrations. In a multivariate model of the whole sample, body fat (BF), waist circumference (WC) and the sex × WC interaction term presented the highest R 2 for variance of CRP (11%), C3 (2%), and Cp (12%), respectively. In regression models separated by sex, BF was the adiposity indicator that explained the variability of CRP in men (13%) and women (7%). WC was the only variable significantly associated with C3 concentrations in women (3%). BF presented the highest partial R 2 for Cp in men (8%) and WC in women (16%).
Conclusion
Our findings indicate a relevant interaction between gender and body fat distribution on the variance of CRP, C3, and Cp concentrations in apparently healthy young adults.



Similar content being viewed by others
References
Moreno-Aliaga MJ, Campión J, Milagro FI, Berjón A, Martínez JA. Adiposity and proinflammatory state: the chicken or the egg. Adipocytes. 2005;1:1–16.
Volp AC, Alfenas Rde C, Costa NM, Minim VP, Stringueta PC, Bressan J. Inflammation biomarkers capacity in predicting the metabolic syndrome. Arq Bras Endocrinol Metabol. 2008;52:537–49.
Zulet MA, Puchau B, Navarro C, Marti A, Martínez JA. Inflammatory biomarkers: the link between obesity and associated pathologies. Nutr Hosp. 2007;22:511–27.
Engström G, Hedblad B, Eriksson KF, Janzon L, Lindgarde F. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes. 2005;54:570–5.
Festa A, D’Agostino R Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25:1407–15.
Kim OY, Shin MJ, Moon J, Chung JH. Plasma ceruloplasmin as a biomarker for obesity: a proteomic approach. Clin Biochem. 2011;44:351–6.
Puchau B, Zulet MA, González de Echavarri A, Navarro-Blasco I, Martínez JA. Selenium intake reduces serum C3, an early marker of metabolic syndrome manifestations, in healthy young adults. Eur J Clin Nutr. 2009;63:858–64.
Choi EY, Park EH, Cheong YS, Rheem I, Park SG, Yoo S. Association of C-reactive protein with the metabolic risk factors among young and middle-aged Koreans. Metabolism. 2006;55:415–21.
Engström G, Hedblad B, Tyden P, Lindgarde F. Inflammation-sensitive plasma proteins are associated with increased incidence of heart failure: a population-based cohort study. Atherosclerosis. 2009;202:617–22.
Wang B, Li Q, Jiang Y, Liu Z, Zhong L, Luo R, et al. Serum complement C3 has a stronger association with insulin resistance than high sensitive C-reactive protein in non-diabetic Chinese. Inflamm Res. 2011;60:63–8.
Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7:147–59.
Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–36.
Hermsdorff HHM, Puchau B, Zulet MA, Martínez JA. Association of body fat distribution with proinflammatory gene expression in peripheral blood mononuclear cells from young adult subjects. OMICS. 2010;14:297–307.
Hermsdorff HHM, Zulet MA, Puchau B, Martínez JA. Central adiposity rather than total adiposity measurements are specifically involved in the inflammatory status from healthy young adults. Inflammation. 2011;34:161–70.
Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009;32:1734–6.
Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–33.
Hermsdorff HHM, Zulet MA, Bressan J, Martínez-González MA, Martínez JA. Impact of caloric restriction and Mediterranean food-based diets on inflammation accompanying obesity and metabolic syndrome features. Obes Metab. 2009;5:69–77.
Vague J. Sexual differentiation. A determinant factor of the forms of obesity. 1947. Obes Res. 1996;4:201–3.
Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–7.
Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89:1307–14.
Leung J, Jayachandran M, Kendall-Thomas J, Behrenbeck T, Araoz P, Miller VM. Pilot study of sex differences in chemokine/cytokine markers of atherosclerosis in humans. Gend Med. 2008;5:44–52.
Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41.
Saltevo J, Vanhala M, Kautiainen H, Kumpusalo E, Laakso M. Gender differences in C-reactive protein, interleukin-1 receptor antagonist and adiponectin levels in the metabolic syndrome: a population-based study. Diabet Med. 2008;25:747–50.
Hermsdorff HHM, Zulet MA, Puchau B, Bressan J, Martínez JA. Association of retinol-binding protein-4 with dietary selenium intake and other lifestyle features in young healthy women. Nutrition. 2009;25:392–9.
Pérez S, Parra MD, Martínez de Morentin B, Rodríguez MC, Martínez JA. Evaluación de la variabilidad intraindividual de la medida de composición corporal mediante bioimpedancia en voluntarias sanas y su relación con el índice de masa corporal y el pliegue tricipital. Enferm Clin. 2005;15:307–14.
World Health Organization (WHO). Programme of nutrition, family and reproductive health. obesity. Preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva, 1997. 1998.
Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97.
Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–80.
Whitworth JA, Chalmers J. World health organisation-international society of hypertension (WHO/ISH) hypertension guidelines. Clin Exp Hypertens. 2004;26:747–52.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Castelli WP. Cholesterol and lipids in the risk of coronary artery disease—the Framingham Heart Study. Can J Cardiol 1988; 4 Suppl A:5A–10A.
Willett WC. Nutritional epidemiology. New York: Oxford University Press; 1998.
Hermsdorff HHM, Monteiro JB. Visceral, subcutaneous or intramuscular fat: where is the problem? Arq Bras Endocrinol Metabol. 2004;48:803–11.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
Hermsdorff HHM, Zulet MA, Abete I, Martínez JA. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine. 2009;36:445–51.
Hermsdorff HHM, Zulet MA, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–9.
Lee IS, Shin G, Choue R. A 12-week regimen of caloric restriction improves levels of adipokines and pro-inflammatory cytokines in Korean women with BMIs greater than 23 kg/m2. Inflamm Res. 2010;59:399–405.
Nishida M, Moriyama T, Sugita Y, Yamauchi-Takihara K. Abdominal obesity exhibits distinct effect on inflammatory and anti-inflammatory proteins in apparently healthy Japanese men. Cardiovasc Diabetol. 2007;6:27.
Santos AC, Lopes C, Guimaraes JT, Barros H. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int J Obes (Lond). 2005;29:1452–6.
Cignarelli M, DePergola G, Picca G, Sciaraffia M, Pannacciulli N, Tarallo M, et al. Relationship of obesity and body fat distribution with ceruloplasmin serum levels. Int J Obes Relat Metab Disord. 1996;20:809–13.
Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–8.
Després JP, Allard C, Tremblay A, Talbot J, Bouchard C. Evidence for a regional component of body fatness in the association with serum lipids in men and women. Metabolism. 1985;34:967–73.
Onat A, Hergenc G, Can G, Kaya Z, Yuksel H. Serum complement C3: a determinant of cardiometabolic risk, additive to the metabolic syndrome, in middle-aged population. Metabolism. 2009;59:628–34.
Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med 2007; 4 Suppl B:S162–S177.
Saltevo J, Kautiainen H, Vanhala M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend Med. 2009;6:463–70.
Johnson PE, Milne DB, Lykken GI. Effects of age and sex on copper absorption, biological half-life, and status in humans. Am J Clin Nutr. 1992;56:917–25.
Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27:2351–9.
Garmizo G, Frauens BJ. Corneal copper deposition secondary to oral contraceptives. Optom Vis Sci. 2008;85:E802–7.
van Rooijen M, Hansson LO, Frostegard J, Silveira A, Hamsten A, Bremme K. Treatment with combined oral contraceptives induces a rise in serum C-reactive protein in the absence of a general inflammatory response. J Thromb Haemost. 2006;4:77–82.
Acknowledgments
We wish to thank the physician Blanca E. Martínez de Morentín, the nurse Salomé Pérez, and the technician Verónica Ciaurriz as well as the nursing assistant Elisângela Lessa for excellent technical assistance in the University of Navarra and Federal University of Viçosa, respectively. We also thank to The Capes Foundation, Ministry of Education of Brazil, as well as the Ministry of Education of Spain for supporting the Interuniversity Cooperation between the Federal University of Viçosa (CAPES/MECD-DGU 109/06) and the University of Navarra (PHB-2005-0119-PC). Finally, this work was supported by the Health Department of the Government of Navarra (22/2007), Línea Especial about Nutrition, Obesity and Health (University of Navarra LE/97), and by the Foundation for Research Support of the State of Minas Gerais (FAPEMIG-CDS 303/06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Claudia Kasserra.
Rights and permissions
About this article
Cite this article
Hermsdorff, H.H., Volp, A.C., Puchau, B. et al. Contribution of gender and body fat distribution to inflammatory marker concentrations in apparently healthy young adults. Inflamm. Res. 61, 427–435 (2012). https://doi.org/10.1007/s00011-011-0429-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0429-z