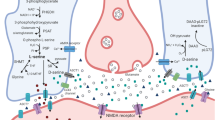
Abstract
Over the past years, accumulating evidence has indicated that d-serine is the endogenous ligand for the glycine-modulatory binding site on the NR1 subunit of N-methyl-d-aspartate receptors in various brain areas. d-Serine is synthesized in glial cells and neurons by the pyridoxal-5′ phosphate-dependent enzyme serine racemase, and it is released upon activation of glutamate receptors. The cellular concentration of this novel messenger is regulated by both serine racemase isomerization and elimination reactions, as well as by its selective degradation catalyzed by the flavin adenine dinucleotide-containing flavoenzyme d-amino acid oxidase. Here, we present an overview of the current knowledge of the metabolism of d-serine in human brain at the molecular and cellular levels, with a specific emphasis on the brain localization and regulatory pathways of d-serine, serine racemase, and d-amino acid oxidase. Furthermore, we discuss how d-serine is involved with specific pathological conditions related to N-methyl-d-aspartate receptors over- or down-regulation.






Similar content being viewed by others
Abbreviations
- Aβ:
-
Amyloid β-peptide
- AD:
-
Alzheimer’s disease
- AMPA:
-
α-Amino-3-hydroxy-5-methylisooxazole-4-propionic acid
- CNS:
-
Central nervous system
- CP:
-
Choroid plexus
- CSF:
-
Cerebrospinal fluid
- DAAO:
-
d-Amino acid oxidase (EC 1.4.3.3)
- d-DOPA:
-
d-3,4-Dihydroxyphenylalanine
- DPFC:
-
Dorsolateral prefrontal cortex
- FAD:
-
Flavin adenine dinucleotide
- Golga3:
-
Golgin subfamily A member
- GRIP:
-
Glutamate receptor interacting protein
- PDZ:
-
PSD95/disc large/ZO-1
- PICK1:
-
Protein interacting with C kinase 1
- PIP2:
-
Phosphatidylinositol(4,5)biphosphate
- PLP:
-
Pyridoxal-5′ phosphate
- NMDAR:
-
N-methyl-d-aspartate receptor
- NO:
-
Nitric oxide
- SR:
-
Serine racemase (EC 5.1.1.18)
References
Hashimoto A, Oka T, Nishikawa T (1995) Extracellular concentration of endogenous free d-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience 66:635–643
Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO Jr, Ferris CD, Snyder SH (1999) Purification of serine racemase: biosynthesis of the neuromodulator d-serine. Proc Natl Acad Sci USA 96:721–725
Schell MJ, Molliver ME, Snyder SH (1995) d-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92:3948–3952
Danysz W, Parsons CG (1998) Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50:597–664
Hashimoto A, Nishikawa T, Oka T, Takahashi K (1993) Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J Neurochem 60:783–786
Schell MJ, Brady RO Jr, Molliver ME, Snyder SH (1997) d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 17:1604–1615
Ribeiro CS, Reis M, Panizzutti R, de Miranda J, Wolosker H (2002) Glial transport of the neuromodulator d-serine. Brain Res 929:202–209
Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G (2005) Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA 102:5606–5611
Martineau M, Galli T, Baux G, Mothet JP (2008) Confocal imaging and tracking of the exocytotic routes for d-serine-mediated gliotransmission. Glia 56:1271–1284
Oliet SH, Mothet JP (2009) Regulation of N-methyl-d-aspartate receptors by astrocytic d-serine. Neuroscience 158:275–283
Yasuda E, Ma N, Semba R (2001) Immunohistochemical demonstration of l-serine distribution in the rat brain. Neuroreport 12:1027–1030
Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H (2006) Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem 281:14151–14162
Williams SM, Diaz CM, Macnab LT, Sullivan RK, Pow DV (2006) Immunocytochemical analysis of d-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia 53:401–411
Dun Y, Mysona B, Itagaki S, Martin-Studdard A, Ganapathy V, Smith SB (2007) Functional and molecular analysis of d-serine transport in retinal Müller cells. Exp Eye Res 84:191–199
Shao Z, Kamboj A, Anderson CM (2009) Functional and immunocytochemical characterization of d-serine transporters in cortical neuron and astrocyte cultures. J Neurosci Res 87:2520–2530
Helboe L, Egebjerg J, Møller M, Thomsen C (2003) Distribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur J Neurosci 18:2227–2238
Matsuo H, Kanai Y, Tokunaga M, Nakata T, Chairoungdua A, Ishimine H, Tsukada S, Ooigawa H, Nawashiro H, Kobayashi Y, Fukuda J, Endou H (2004) High affinity d- and l-serine transporter Asc-1: cloning and dendritic localization in the rat cerebral and cerebellar cortices. Neurosci Lett 358:123–126
Xie X, Dumas T, Tang L, Brennan T, Reeder T, Thomas W, Klein RD, Flores J, O’Hara BF, Heller HC, Franken P (2005) Lack of the alanine–serine–cysteine transporter 1 causes tremors, seizures, and early postnatal death in mice. Brain Res 1052:212–221
Schell MJ (2004) The N-methyl d-aspartate receptor glycine site and d-serine metabolism: an evolutionary perspective. Philos Trans R Soc Lond B Biol Sci 359:943–964
Martineau M, Baux G, Mothet JP (2006) d-Serine signalling in the brain: friend and foe. Trends Neurosci 29:481–491
Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S (2003) Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc Natl Acad Sci USA 100:15194–15199
Junjaud G, Rouaud E, Turpin F, Mothet JP, Billard JM (2006) Age-related effects of the neuromodulator d-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. J Neurochem 98:1159–1166
Panatier A, Theodosis DT, Mothet J-P, Touquet B, Pollegioni L, Poulain DA, Oliet SHR (2006) Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell 125:775–784
Strísovský K, Jirásková J, Barinka C, Majer P, Rojas C, Slusher BS, Konvalinka J (2003) Mouse brain serine racemase catalyzes specific elimination of l-serine to pyruvate. FEBS Lett 535:44–48
Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, Li P, Toney MD, Kartvelishvily E, Wolosker H (2005) Serine racemase modulates intracellular d-serine levels through an alpha, beta-elimination activity. J BiolChem 280:1754–1763
Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96:13409–13414
Panizzutti R, De Miranda J, Ribeiro CS, Engelender S, Wolosker H (2001) A new strategy to decrease N-methyl-d-aspartate (NMDA) receptor coactivation: inhibition of d-serine synthesis by converting serine racemase into an eliminase. Proc Natl Acad Sci USA 98:5294–5299
De Miranda J, Panizzutti R, Foltyn VN, Wolosker H (2002) Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-d-aspartate (NMDA) receptor coagonist d-serine. Proc Natl Acad Sci USA 99:14542–14547
Cook SP, Galve-Roperh I, Martínez del Pozo A, Rodríguez-Crespo I (2002) Direct calcium binding results in activation of brain serine racemase. J Biol Chem 277:27782–27792
Neidle A, Dunlop DS (2002) Allosteric regulation of mouse brain serine racemase. Neurochem Res 27:1719–1724
Dunlop DS, Neidle A (2005) Regulation of serine racemase activity by amino acids. Brain Res Mol Brain Res 133:208–214
Strísovský K, Jirásková J, Mikulová A, Rulísek L, Konvalinka J (2005) Dual substrate and reaction specificity in mouse serine racemase: identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the beta-eliminase activity. Biochemistry 44:13091–13100
De Miranda J, Santoro A, Engelender S, Wolosker H (2000) Human serine racemase: molecular cloning, genomic organization and functional analysis. Gene 256:183–188
Xia M, Liu Y, Figueroa DJ, Chiu CS, Wei N, Lawlor AM, Lu P, Sur C, Koblan KS, Connolly TM (2004) Characterization and localization of a human serine racemase. Brain Res Mol Brain Res 125:96–104
Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, Nakamura K, Shimizu E, Itokawa M, Mori N, Iyo M, Yoshikawa T (2005) Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and d-serine levels. Biol Psychiatry 57:1493–1503
Hoffman HE, Jirásková J, Ingr M, Zvelebil M, Konvalinka J (2009) Recombinant human serine racemase: enzymologic characterization and comparison with its mouse ortholog. Protein Expr Purif 63:62–67
Baumgart F, Mancheño JM, Rodríguez-Crespo I (2007) Insights into the activation of brain serine racemase by the multi-PDZ domain glutamate receptor interacting protein, divalent cations and ATP. FEBS J 274:4561–4571
Baumgart F, Rodríguez-Crespo I (2008) d-amino acids in the brain: the biochemistry of brain serine racemase. FEBS J 275:3538–3545
Pilone MS (2000) d-Amino acid oxidase: new findings. Cell Mol Life Sci 57:1732–1747
Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G (2007) Physiological functions of d-amino acid oxidases: from yeast to humans. Cell Mol Life Sci 64:1373–1394
Krebs HA (1935) Metabolism of amino-acids: deamination of amino-acids. Biochem J 29:1620–1644
Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K (1992) The presence of free d-serine in rat brain. FEBS Lett 296:33–36
Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K (1993) Free d-serine, d-aspartate and d-alanine in central nervous system and serum in mutant mice lacking d-amino acid oxidase. Neurosci Lett 152:33–36
Nagata Y, Yamamoto K, Shimojo T, Konno R, Yasumura Y, Akino T (1992) The presence of free d-alanine, d-proline and d-serine in mice. Biochim Biophys Acta 1115:208–211
Nagata Y, Horiike K, Maeda T (1994) Distribution of free d-serine in vertebrate brains. Brain Res 634:291–295
Konno R (2001) Assignment of d-amino-acid oxidase gene to a human and a mouse chromosome. Amino Acids 20:401–408
Momoi K, Fukui K, Watanabe F, Miyake Y (1988) Molecular cloning and sequence analysis of cDNA encoding human kidney d-amino acid oxidase. FEBS Lett 238:180–184
Romano D, Molla G, Pollegioni L, Marinelli F (2009) Optimization of human d-amino acid oxidase expression in Escherichia coli. Protein Expr Purif 68:72–78
Molla G, Sacchi S, Bernasconi M, Pilone MS, Fukui K, Pollegioni L (2006) Characterization of human d-amino acid oxidase. FEBS Lett 580:2358–2364
Caldinelli L, Molla G, Sacchi S, Pilone MS, Pollegioni L (2009) Relevance of weak flavin binding in human d-amino acid oxidase. Protein Sci 18:801–810
Kawazoe T, Tsuge H, Imagawa T, Aki K, Kuramitsu S, Fukui K (2007) Structural basis of d-DOPA oxidation by d-amino acid oxidase: alternative pathway for dopamine biosynthesis. Biochem Biophys Res Commun 355:385–391
Kawazoe T, Tsuge H, Pilone MS, Fukui K (2006) Crystal structure of human d-amino acid oxidase: context-dependent variability of the backbone conformation of the VAAGL hydrophobic stretch located at the si-face of the flavin ring. Protein Sci 15:2708–2717
Mattevi A, Vanoni MA, Todone F, Rizzi M, Teplyakov A, Coda A, Bolognesi M, Curti B (1996) Crystal structure of d-amino acid oxidase: a case of active site mirror-image convergent evolution with flavocytochrome b2. Proc Natl Acad Sci USA 93:7496–7501
Mizutani H, Miyahara I, Hirotsu K, Nishina Y, Shiga K, Setoyama C, Miura R (1996) Three-dimensional structure of porcine kidney d-amino acid oxidase at 3.0 Å resolution. J Biochem 120:14–17
Shoji K, Mariotto S, Ciampa AR, Suzuki H (2006) Regulation of serine racemase activity by d-serine and nitric oxide in human glioblastoma cells. Neurosci Lett 392:75–78
Shoji K, Mariotto S, Ciampa AR, Suzuki H (2006) Mutual regulation between serine and nitric oxide metabolism in human glioblastoma cells. Neurosci Lett 394:163–167
Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH (2007) Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of d-serine formation. Proc Natl Acad Sci USA 104:2950–2955
Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH (2005) Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA 102:2105–2110
Hung AY, Sheng M (2002) PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702
Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J, Yamada T, Ozeki Y, Kawahara R, Okawa M, Huganir RL, Ujike H, Snyder SH, Sawa A (2006) Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry 11:150–157
Pan L, Wu H, Shen C, Shi Y, Jin W, Xia J, Zhang M (2007) Clustering and synaptic targeting of PICK1 requires direct interaction between the PDZ domain and lipid membranes. EMBO J 26:4576–4587
Hikida T, Mustafa AK, Maeda K, Fujii K, Barrow RK, Saleh M, Huganir RL, Snyder SH, Hashimoto K, Sawa A (2008) Modulation of d-serine levels in brains of mice lacking PICK1. Biol Psychiatry 63:997–1000
Dumin E, Bendikov I, Foltyn VN, Misumi Y, Ikehara Y, Kartvelishvily E, Wolosker H (2006) Modulation of d-serine levels via ubiquitin-dependent proteasomal degradation of serine racemase. J Biol Chem 281:20291–20302
Balan L, Foltyn VN, Zehl M, Dumin E, Dikopoltsev E, Knoh D, Ohno Y, Kihara A, Jensen ON, Radzishevsky IS, Wolosker H (2009) Feedback inactivation of d-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc NatlAcad Sci USA 106:7589–7594
Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH (2009) Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci USA 106:2921–2926
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D (2002) Genetic and physiological data implicating the new human gene G72 and the gene for d-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99:13675–13680
Liu X, He G, Wang X, Chen Q, Qian X, Lin W, Li D, Gu N, Feng G, He L (2004) Association of DAAO with schizophrenia in the Chinese population. Neurosci Lett 369:228–233
Molla G, Bernasconi M, Sacchi S, Pilone MS, Pollegioni L (2006) Expression in Escherichia coli and in vitro refolding of the human protein pLG72. Protein Expr Purif 46:150–155
Sacchi S, Bernasconi M, Martineau M, Mothet JP, Ruzzene M, Pilone MS, Pollegioni L, Molla G (2008) pLG72 modulates intracellular d-serine levels through its interaction with d-amino acid oxidase: effect on schizophrenia susceptibility. J Biol Chem 283:22244–22256
Kvajo M, Dhilla A, Swor DE, Karayiorgou M, Gogos JA (2008) Evidence implicating the candidate schizophrenia/bipolar disorder susceptibility gene G72 in mitochondrial function. Mol Psychiatry 13:685–696
Otte DM, Bilkei-Gorzó A, Filiou MD, Turck CW, Yilmaz O, Holst MI, Schilling K, Abou-Jamra R, Schumacher J, Benzel I, Kunz WS, Beck H, Zimmer A (2009) Behavioral changes in G72/G30 transgenic mice. Eur Neuropsychopharmacol 19:339–348
Konno R (2003) Rat cerebral serine racemase: amino acid deletion and truncation at carboxy terminus. Neurosci Lett 349:111–114
Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW (2004) Induction of serine racemase expression and d-serine release from microglia by amyloid beta-peptide. J Neuroinflammation 1:2. doi:10.1186/1742-2094-1-2
Yoshikawa M, Nakajima K, Takayasu N, Noda S, Sato Y, Kawaguchi M, Oka T, Kobayashi H, Hashimoto A (2006) Expression of the mRNA and protein of serine racemase in primary cultures of rat neurons. Eur J Pharmacol 548:74–76
Yoshikawa M, Takayasu N, Hashimoto A, Sato Y, Tamaki R, Tsukamoto H, Kobayashi H, Noda S (2007) The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch Histol Cytol 70:127–134
Dun Y, Duplantier J, Roon P, Martin PM, Ganapathy V, Smith SB (2008) Serine racemase expression and d-serine content are developmentally regulated in neuronal ganglion cells of the retina. J Neurochem 104:970–978
Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF (2003) d-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA 100:6789–6794
Wolosker H, Dumin E, Balan L, Foltyn VN (2008) d-amino acids in the brain: d-serine in neurotransmission and neurodegeneration. FEBS J 275:3514–3526
Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H (2008) Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol 510:641–654
Hepner F, Pollak A, Ulfig N, Yae-Kyung M, Lubec G (2005) Mass spectrometrical analysis of human serine racemase in foetal brain. J Neural Transm 112:805–811
Neims AH, Zieverink WD, Smilack JD (1966) Distribution of d-amino acid oxidase in bovine and human nervous tissues. J Neurochem 13:163–168
Goldstein DB (1966) d-amino acid oxidase in brain: distribution in several species and inhibition by pentobarbitone. J Neurochem 13:1011–1016
Arnold G, Liscum L, Holtzman E (1979) Ultrastructural localization of d-amino acid oxidase in microperoxisomes of the rat nervous system. J Histochem Cytochem 27:735–745
Horiike K, Tojo H, Arai R, Yamano T, Nozaki M, Maeda T (1987) Localization of d-amino acid oxidase in Bergmann glial cells and astrocytes of rat cerebellum. Brain Res Bull 19:587–596
Horiike K, Tojo H, Arai R, Nozaki M, Maeda T (1994) d-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res 652:297–303
Kappor R, Kapoor V (1997) Distribution of d-amino acid oxidase (DAO) activity in the medulla and thoracic spinal cord of the rat: implications for a role for d-serine in autonomic function. Brain Res 771:351–355
Moreno S, Nardacci R, Cimini A, Cerù MP (1999) Immunocytochemical localization of d-amino acid oxidase in rat brain. J Neurocytol 28:169–185
Urai Y, Jinnouchi O, Kwak KT, Suzue A, Nagahiro S, Fukui K (2002) Gene expression of d-amino acid oxidase in cultured rat astrocytes: regional and cell type specific expression. Neurosci Lett 324:101–104
Cristiano L, Bernardo A, Cerù MP (2001) Peroxisome proliferator-activated receptors (PPARs) and peroxisomes in rat cortical and cerebellar astrocytes. J Neurocytol 30:671–683
Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW (2007) d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci 26:1657–1669
Ono K, Shishido Y, Park HK, Kawazoe T, Iwana S, Chung SP, Abou El-Magd RM, Yorita K, Okano M, Watanabe T, Sano N, Bando Y, Arima K, Sakai T, Fukui K (2009) Potential pathophysiological role of d-amino acid oxidase in schizophrenia: immunohistochemical and in situ hybridization study of the expression in human and rat brain. J Neural Transm 116:1335–1347
Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, Patel S (2007) Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating d-serine reuptake in the mouse CNS. Eur J Neurosci 25:1757–1766
Morikawa A, Hamase K, Inoue T, Konno R, Niwa A, Zaitsu K (2001) Determination of free d-aspartic acid, d-serine and d-alanine in the brain of mutant mice lacking d-amino acid oxidase activity. J Chromatogr B Biomed Sci Appl 757:119–125
Wu S, Basile AS, Barger SW (2007) Induction of serine racemase expression and d-serine release from microglia by secreted amyloid precursor protein (sAPP). Curr Alzheimer Res 4:243–251
Inoue R, Hashimoto K, Harai T, Mori H (2008) NMDA- and beta-amyloid1–42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci 28:14486–14491
Sasabe J, Chiba T, Yamada M, Okamoto K, Nishimoto I, Matsuoka M, Aiso S (2007) d-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J 26:4149–4159
Katsuki H, Nonaka M, Shirakawa H, Kume T, Akaike A (2004) Endogenous d-serine is involved in induction of neuronal death by N-methyl-d-aspartate and simulated ischemia in rat cerebrocortical slices. J Pharmacol Exp Ther 311:836–844
Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81:740–749
Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT (2006) Neurobiology of schizophrenia. Neuron 52:139–153
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308
Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH, Roder JC (2009) Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet 18:3227–3243
Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT (2009) Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 14:719–727
Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M (2003) Decreased serum levels of d-serine in patients with schizophrenia: evidence in support of the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 60:572–576
Bendikov I, Nadir S, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G (2007) A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr Res 90:41–51
Steffek AE, Haroutunian V, Meador-Woodruff JH (2006) Serine racemase protein expression in cortex and hippocampus in schizophrenia. Neuroreport 17:1181–1185
Burnet PW, Eastwood SL, Bristow GC, Godlewska BR, Sikka P, Walker M, Harrison PJ (2008) d-Amino acid oxidase activity and expression are increased in schizophrenia. Mol Psychiatry 13:658–660
Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R (2008) Increased brain d-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res 101:76–83
Ohi K, Hashimoto R, Yasuda Y, Yoshida T, Takahashi H, Iike N, Fukumoto M, Takamura H, Iwase M, Kamino K, Ishii R, Kazui H, Sekiyama R, Kitamura Y, Azechi M, Ikezawa K, Kurimoto R, Kamagata E, Tanimukai H, Tagami S, Morihara T, Ogasawara M, Okochi M, Tokunaga H, Numata S, Ikeda M, Ohnuma T, Ueno S, Fukunaga T, Tanaka T, Kudo T, Arai H, Ohmori T, Iwata N, Ozaki N, Takeda M (2009) Association study of the G72 gene with schizophrenia in a Japanese population: a multicenter study. Schizophr Res 109:80–85
Opgen-Rhein C, Lencz T, Burdick KE, Neuhaus AH, DeRosse P, Goldberg TE, Malhotra AK (2008) Genetic variation in the DAOA gene complex: impact on susceptibility for schizophrenia and on cognitive performance. Schizophr Res 103:169–177
Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, Coppola R, Egan MF, Weinberger DR (2006) The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology 31:2022–2032
Ohnuma T, Shibata N, Maeshima H, Baba H, Hatano T, Hanzawa R, Arai H (2009) Association analysis of glycine- and serine-related genes in a Japanese population of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33:511–518
Goltsov AY, Loseva JG, Andreeva TV, Grigorenko AP, Abramova LI, Kaleda VG, Orlova VA, Moliaka YK, Rogaev EI (2006) Polymorphism in the 5′-promoter region of serine racemase gene in schizophrenia. Mol Psychiatry 11:325–326
Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, Hamase K, Zaitsu K, Kuroda S (2007) A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry 61:1200–1203
Yoshikawa M, Shinomiya T, Takayasu N, Tsukamoto H, Kawaguchi M, Kobayashi H, Oka T, Hashimoto A (2008) Long-term treatment with morphine increases the d-serine content in the rat brain by regulating the mRNA and protein expressions of serine racemase and d-amino acid oxidase. J Pharmacol Sci 107:270–276
Turpin FR, Potier B, Dulong JR, Sinet PM, Alliot J, Oliet SH, Dutar P, Epelbaum J, Mothet JP, Billard JM (2009) Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2009.09.001. Epub ahead of print
Acknowledgments
This work was supported by grants from Fondo di Ateneo per la Ricerca to L. Pollegioni and S. Sacchi, and from Fondazione CARIPLO to L. Pollegioni. We are grateful for the support of Consorzio Interuniversitario per le Biotecnologie and the Centro di Ricerca in Biotecnologie per la Salute Umana (Università degli studi dell’Insubria). The authors are grateful to all members of their laboratory and particularly to Mirella Pilone, Gianluca Molla (even for help in preparing Fig. 3), Laura Caldinelli, and Pamela Cappelletti for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pollegioni, L., Sacchi, S. Metabolism of the neuromodulator d-serine. Cell. Mol. Life Sci. 67, 2387–2404 (2010). https://doi.org/10.1007/s00018-010-0307-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0307-9