Abstract
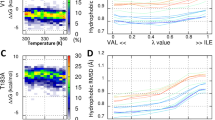
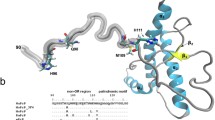
The conversion of the cellular prion protein (PrPC) into its disease-associated form (PrPSc) involves a major conformational change and the accumulation of sulfoxidized methionines. Computational and synthetic approaches have shown that this change in the polarity of M206 and M213 impacts the C-terminal domain native α-fold allowing the flexibility required for the structural conversion. To test the effect in the full-length molecule with site-specificity, we have generated M-to-S mutations. Molecular dynamics simulations show that the replacement indeed perturbs the native state. When this mutation is placed at the conserved methionines of HaPrP(23–231), only substitutions at the Helix-3 impair the α-fold, stabilizing a non-native state with perturbed secondary structure, loss of native tertiary contacts, increased surface hydrophobicity, reduced thermal stability and an enhanced tendency to aggregate into protofibrillar polymers. Our work supports that M206 and M213 function as α-fold gatekeepers and suggests that their redox state regulate misfolding routes.






Similar content being viewed by others
Abbreviations
- PrPC :
-
Cellular prion protein
- PrPSc :
-
Disease-related form of PrP
- HuPrP(125–229):
-
Polypeptide chain representing the globular domain of the human PrP
- HaPrP(23–231):
-
Polypeptide chain representing the mature chain of the hamster PrP
- MD:
-
Molecular dynamics
- RWISP:
-
Root weighted square inner product
- CP:
-
Communication propensity
- CD:
-
Circular dichroism
- DLS:
-
Dynamic light scattering
- Rh :
-
Hydrodynamic radius
- ThT:
-
Thioflavin T
- AFM:
-
Atomic force microscopy
References
Prusiner SB (2001) Shattuck lecture: neurodegenerative diseases and prions. N Engl J Med 344:1516–1526
Aguzzi A, Baumann F, Bremer J (2008) The prion’s elusive reason for being. Annu Rev Neurosci 31:439–477
Oesch B, Westaway D, Wälchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE, Prusiner SB, Weissmann C (1985) A cellular gene encodes scrapie PrP 27–30 protein. Cell 40:735–746
Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, Prusiner SB (1986) Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci USA 83:2310–2314
Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS (1991) Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30:7672–7680
McKinley MP, Meyer RK, Kenaga L, Rahbar F, Cotter R, Serban A, Prusiner SB (1991) Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J Virol 65:1340–1351
Gasset M, Baldwin MA, Lloyd DH, Gabriel JM, Holtzman DM, Cohen F, Fletterick R, Prusiner SB (1992) Predicted alpha-helical regions of the prion protein when synthesized as peptides form amyloid. Proc Natl Acad Sci USA 189:10940–10944
Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R, Cohen F, Prusiner SB (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA 90:10962–10966
Baskakov IV, Breydo L (2007) Converting the prion protein: what makes the protein infectious. Biochim Biophys Acta 1772:692–703
Sigurdson CJ, Nilsson KP, Hornemann S, Heikenwalder M, Manco G, Schwarz P, Ott D, Rülicke T, Liberski PP, Julius C, Falsig J, Stitz L, Wüthrich K, Aguzzi A (2009) De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci USA 106:304–309
Colby DW, Giles K, Legname G, Wille H, Baskakov IV, Dearmond SJ, Prusiner SB (2009) Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA 106:20417–20422
Stahl N, Baldwin MA, Teplow DB, Hood L, Gibson BW, Burlingame AL, Prusiner SB (1993) Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry 32:1991–2002
Canello T, Engelstein R, Moshel O, Xanthopoulos K, Juanes ME, Langeveld J, Sklaviadis T, Gasset M, Gabizon R (2008) Methionine sulfoxides on PrPSc: a prion-specific covalent signatura. Biochemistry 47:8866–8873
Colombo G, Meli M, Morra G, Gabizon R, Gasset M (2009) Methionine sulfoxides on prion protein Helix-3 switch on the alpha-fold destabilization required for conversion. PLoS One 4(1):e4296
Wolschner C, Giese A, Kretzschmar HA, Huber R, Moroder L, Budisa N (2009) Design of anti- and pro-aggregation variants to assess the effects of methionine oxidation in human prion protein. Proc Natl Acad Sci USA 106:7756–7761
Kelly JW (1998) The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol 8:101–106
Eisenberg D, Nelson R, Sawaya MR, Balbirnie M, Sambashivan S, Ivanova MI, Madsen AØ, Riekel C (2006) The structural biology of protein aggregation diseases: fundamental questions and some answers. Acc Chem Res 39:568–575
Nelson R, Eisenberg D (2006) Structural models of amyloid-like fibrils. Adv Protein Chem 73:235–282
Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJ, McFarlane HT, Madsen AØ, Riekel C, Eisenberg D (2007) Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447:453–457
Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I (1993) Microtubule-associated protein tau: abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268:24374–24384
Iqbal K, Liu F, Gong CX, Alonso AC, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118:53–69
Sun Q, Gamblin TC (2009) Pseudohyperphosphorylation causing AD-like changes in tau has significant effects on its polymerization. Biochemistry 48:6002–6011
Stadtman ER (2006) Protein oxidation and aging. Free Radic Res 40:1250–1258
Oien DB, Moskovitz J (2008) Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr Top Dev Biol 80:93–133
Hernández F, Nido JD, Avila J, Villanueva N (2009) GSK3 inhibitors and disease. Mini Rev Med Chem 9:1024–1029
Virshup DM, Shenolikar S (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33:537–545
Ostapchenko VG, Makarava N, Savtchenko R, Baskakov IV (2008) The polybasic N-terminal region of the prion protein controls the physical properties of both the cellular and fibrillar forms of PrP. J Mol Biol 383:1210–1224
Dado GP, Gellman SH (1993) Redox control of secondary structure in a designed peptide. J Am Chem Soc 115:12609–12610
Schenck HL, Schenck HL, Dado GP, Gellman SH (1996) Redox-triggered secondary structure changes in the aggregated states of a designed methionine-rich peptide. J Am Chem Soc 118:12487–12494
Binger KJ, Griffin MD, Howlett GJ (2008) Methionine oxidation inhibits assembly and promotes disassembly of apolipoprotein C-II amyloid fibrils. Biochemistry 47:10208–10217
Wiltzius JJ, Landau M, Nelson R, Sawaya MR, Apostol MI, Goldschmidt L, Soriaga AB, Cascio D, Rajashankar K, Eisenberg D (2009) Molecular mechanisms for protein-encoded inheritance. Nat Struct Mol Biol 16:973–978
Vriend G (1990) What if: a molecular modeling and drug design program. J Mol Graph 8:52–56
James TL, Liu H, Ulyanov NB, Farr-Jones S, Zhang H, Donne DG, Kaneko K, Groth D, Mehlhorn I, Prusiner SB, Cohen FE (1997) Solution structure of a 142-residue recombinant prion protein corresponding to the infectious fragment of the scrapie isoform. Proc Natl Acad Sci USA 94:10086–10091
González-Iglesias R, Pajares MA, Ocal C, Espinosa JC, Oesch B, Gasset M (2002) Prion protein interaction with glycosaminoglycan occurs with the formation of oligomeric complexes stabilized by Cu(II) bridges. J Mol Biol 319:527–540
Pace CN, Scholtz JM (1997) Measuring the conformational stability of a protein. In: Creighton (ed) Protein structure, a practical approach. IRL Press, Oxford, pp 299–321
Becktel WJ, Schellman JA (1987) Protein stability curves. Biopolymers 26:1859–1877
Tadeo X, López-Méndez B, Castaño D, Trigueros T, Millet O (2009) Protein stabilization and the Hofmeister effect: the role of hydrophobic solvation. Biophys J 97:2595–2603
González-Iglesias R, Elvira G, Rodríguez-Navarro JA, Vélez M, Calero M, Pajares MA, Gasset M (2004) Cu2+ binding triggers alphaBoPrP assembly into insoluble laminar polymers. FEBS Lett 556:161–166
Bishop MF, Ferrone FA (1984) Kinetics of nucleation-controlled polymerization: a perturbation treatment for use with a secondary pathway. Biophys J 46:631–644
Amadei A, Linssen ABM, Berendsen HJC (1993) Essential dynamics of proteins. Proteins 17:412–425
Carnevale V, Pontiggia F, Micheletti C (2007) Structural and dynamical alignment of enzymes with partial structural similarity. J Phys Condens Matter 19. doi:10.1088/0953-8984/19/28/285206
Lysek DA, Schorn C, Nivon LG, Esteve-Moya V, Christen B, Calzolai L, von Schroetter C, Fiorito F, Herrmann T, Güntert P, Wüthrich K (2005) Prion protein NMR structures of cats, dogs, pigs, and sheep. Proc Natl Acad Sci USA 102:640–645
Hawe A, Sutter M, Jiskoot W (2008) Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res 25:1487–1499
Creighton TE (1990) Protein folding. Biochem J 270:1–16
Jahn TR, Parker MJ, Homans SW, Radford SE (2006) Amyloid formation under physiological conditions proceeds via a native-like folding intermediate. Nat Struct Mol Biol 13:195–201
Goldsbury C, Green J (2005) Time-lapse atomic force microscopy in the characterization of amyloid-like fibril assembly and oligomeric intermediates. Methods Mol Biol 299:103–128
Campioni S, Mossuto MF, Torrassa S, Calloni G, de Laureto PP, Relini A, Fontana A, Chiti F (2008) Conformational properties of the aggregation precursor state of HypF-N. J Mol Biol 379:554–567
Kaimann T, Metzger S, Kuhlmann K, Brandt B, Birkmann E, Höltje HD, Riesner D (2008) Molecular model of an alpha-helical prion protein dimer and its monomeric subunits as derived from chemical cross-linking and molecular modeling calculations. J Mol Biol 376:582–596
Kuwajima K (1989) The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins 6:87–103
Sanz JM, Fersht AR (1993) Rationally designing the accumulation of a folding intermediate of barnase by protein engineering. Biochemistry 32:13584–13592
Pettersson-Kastberg J, Aits S, Gustafsson L, Mossberg A, Storm P, Trulsson M, Persson F, Mok KH, Svanborg C (2009) Can misfolded proteins be beneficial? The HAMLET case. Ann Med 41:162–176
Gambin Y, Schug A, Lemke EA, Lavinder JJ, Ferreon AC, Magliery TJ, Onuchic JN, Deniz AA (2009) Direct single-molecule observation of a protein living in two opposed native structures. Proc Natl Acad Sci USA 106:10153–10158
Silva JL, Vieira TC, Gomes MP, Bom AP, Lima LM, Freitas MS, Ishimaru D, Cordeiro Y, Foguel D (2009) Ligand binding and hydration in protein misfolding: insights from studies of prion and p53 tumor suppressor proteins (dagger). Acc Chem Res 43:271–279
Kremer W, Kachel N, Kuwata K, Akasaka K, Kalbitzer HR (2007) Species-specific differences in the intermediate states of human and Syrian hamster prion protein detected by high pressure NMR spectroscopy. J Biol Chem 282:22689–22698
Hou L, Kang I, Marchant RE, Zagorski MG (2002) Methionine 35 oxidation reduces fibril assembly of the amyloid abeta-(1–42) peptide of Alzheimer’s disease. J Biol Chem 277:40173–40176
Bitan G, Tarus B, Vollers SS, Lashuel HA, Condron MM, Straub JE, Teplow DB (2003) A molecular switch in amyloid assembly: Met35 and amyloid beta-protein oligomerization. J Am Chem Soc 125:15359–15365
Requena JR, Dimitrova MN, Legname G, Teijeira S, Prusiner SB, Levine RL (2004) Oxidation of methionine residues in the prion protein by hydrogen peroxide. Arch Biochem Biophys 432:188–195
Breydo L, Bocharova OV, Makarava N, Salnikov VV, Anderson M, Baskakov IV (2005) Methionine oxidation interferes with conversion of the prion protein into the fibrillar proteinase K-resistant conformation. Biochemistry 44:15534–15543
Hart T, Hosszu LL, Trevitt CR, Jackson GS, Waltho JP, Collinge J, Clarke AR (2009) Folding kinetics of the human prion protein probed by temperature jump. Proc Natl Acad Sci USA 106:5651–5656
Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC (2010) Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J 29:251–262
Green KM, Browning SR, Seward TS, Jewell JE, Ross DL, Green MA, Williams ES, Hoover EA, Telling GC (2008) The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J Gen Virol 89:598–608
Acknowledgments
This work was supported by grants SAF2006-00418 (MG) and BFU2009-07971 (MG) from the Ministerio de Ciencia e Innovación, FOOD-CT-2004-506579 (MG, RG) from the EC and PI101209 (MG) from the Fundación Cien. SL is supported by a FPI-PhD fellowship from the Ministerio de Ciencia e Innovación. We gratefully acknowledge the advice of Dr. Angel Cuesta in relation to the AFM experiments and the technical support of Lara Reviejo and Rosa Sánchez.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Lisa and M. Meli contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lisa, S., Meli, M., Cabello, G. et al. The structural intolerance of the PrP α-fold for polar substitution of the helix-3 methionines. Cell. Mol. Life Sci. 67, 2825–2838 (2010). https://doi.org/10.1007/s00018-010-0363-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0363-1