Abstract
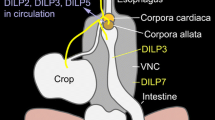
Insulin signaling regulates lifespan, reproduction, metabolic homeostasis, and resistance to stress in the adult organism. In Drosophila, there are seven insulin-like peptides (DILP1–7). Three of these (DILP2, 3 and 5) are produced in median neurosecretory cells of the brain, designated IPCs. Previous work has suggested that production or release of DILPs in IPCs can be regulated by a factor secreted from the fat body as well as by neuronal GABA or short neuropeptide F. There is also evidence that serotonergic neurons may regulate IPCs. Here, we investigated mechanisms by which serotonin may regulate the IPCs. We show that the IPCs in adult flies express the 5-HT1A, but not the 5-HT1B or 5-HT7 receptors, and that processes of serotonergic neurons impinge on the IPC branches. Knockdown of 5-HT1A in IPCs by targeted RNA interference (RNAi) leads to increased sensitivity to heat, prolonged recovery after cold knockdown and decreased resistance to starvation. Lipid metabolism is also affected, but no effect on growth was seen. Furthermore, we show that DILP2-immunolevels in IPCs increase after 5-HT1A knockdown; this is accentuated by starvation. Heterozygous 5-HT1A mutant flies display the same phenotype in all assays, as seen after targeted 5-HT1A RNAi, and flies fed the 5-HT1A antagonist WAY100635 display reduced lifespan at starvation. Our findings suggest that serotonin acts on brain IPCs via the 5-HT1A receptor, thereby affecting their activity and probably insulin signaling. Thus, we have identified a second inhibitory pathway regulating IPC activity in the Drosophila brain.










Similar content being viewed by others
Abbreviations
- 5-HT:
-
5-hydroxytryptamine
- 5-HTP:
-
5-hydroxytryptophan
- BDSC:
-
Bloomington Drosophila stock center
- CNS:
-
Central nervous systems
- DILP:
-
Drosophila insulin-like peptide
- GABA:
-
Gamma-aminobutyric acid
- GFP:
-
Green fluorescent protein
- GIRK:
-
G-protein-coupled inwardly rectifying potassium channel
- GPCR:
-
G-protein-coupled receptor
- IPCs:
-
Insulin- producing cells
- MNCs:
-
Median neurosecretory cells
- NPF:
-
Neuropeptide F
- NS3:
-
Nucleostemin 3
- OAMB:
-
Octopamine receptor (mushroom bodies)
- PCR:
-
Polymerase chain reaction
- PKA:
-
Protein kinase A
- RNAi:
-
RNA interference
- sNPF:
-
Short neuropeptide F
- VDRC:
-
Vienna Drosophila RNAi center
References
Baker KD, Thummel CS (2007) Diabetic larvae and obese flies—emerging studies of metabolism in Drosophila. Cell Metab 6:257–266
Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R et al (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11:213–221
Giannakou ME, Partridge L (2007) Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci 32:180–188
Géminard G, Arquier N, Layalle S, Bourouis M, Slaidina M et al (2006) Control of metabolism and growth through insulin-like peptides in Drosophila. Diabetes 55:S5–S8
Kenyon C (2010) A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann N Y Acad Sci 1204:156–162
Teleman AA (2010) Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425:13–26
Wu Q, Brown MR (2006) Signaling and function of insulin-like peptides in insects. Annu Rev Entomol 51:1–24
Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J (1995) The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J 14:3373–3384
Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L (2010) Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 6:e1000857
Slaidina M, Delanoue R, Grönke S, Partridge L, Leopold P (2009) A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17:874–884
Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J et al (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102:3105–3110
Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM et al (2001) A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292:107–110
Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM et al (2010) DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell 9:336–346
Zhang H, Liu J, Li CR, Momen B, Kohanski RA et al (2009) Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc Natl Acad Sci USA 106:19617–19622
Cao C, Brown MR (2001) Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res 304:317–321
Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296:1118–1120
Broughton S, Alic N, Slack C, Bass T, Ikeya T et al (2008) Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 3:e3721
Ikeya T, Galic M, Belawat P, Nairz K, Hafen E (2002) Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12:1293–1300
Geminard C, Rulifson EJ, Leopold P (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10:199–207
Lee KS, You KH, Choo JK, Han YM, Yu K (2004) Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem 279:50781–50789
Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ et al (2008) Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol 10:468–475
Crocker A, Shahidullah M, Levitan IB, Sehgal A (2010) Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65:670–681
Enell LE, Kapan N, Söderberg JA, Kahsai L, Nässel DR (2010) Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila. PLoS ONE 5:e15780
Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP (2008) A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes Dev 22:1877–1893
Nichols DE, Nichols CD (2008) Serotonin receptors. Chem Rev 108:1614–1641
Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E et al (1990) Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci USA 87:8940–8944
Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R (1992) A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J 11:7–17
Blenau W, Thamm M (2011) Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies. Lessons from Drosophila melanogaster and Apis mellifera. Arthropod Struct Dev (in press)
Yuan Q, Lin F, Zheng X, Sehgal A (2005) Serotonin modulates circadian entrainment in Drosophila. Neuron 47:115–127
Yuan Q, Joiner WJ, Sehgal A (2006) A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol 16:1051–1062
Nichols CD (2007) 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Dev Neurobiol 67:752–763
Johnson O, Becnel J, Nichols CD (2009) Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience 158:1292–1300
Becnel J, Johnson O, Luo J, Nässel DR, Nichols CD (2011) The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE 6(6):e20800
Alekseyenko OV, Lee C, Kravitz EA (2010) Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5:e10806
Daubert EA, Condron BG (2010) Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci 33:424–434
Dierick HA, Greenspan RJ (2007) Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 39:678–682
Rodriguez Moncalvo VG, Campos AR (2009) Role of serotonergic neurons in the Drosophila larval response to light. BMC Neurosci 10:66
Dacks AM, Green DS, Root CM, Nighorn AJ, Wang JW (2009) Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J Neurogenet 23:366–377
Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A et al (2008) Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci USA 105:5579–5584
Wu Q, Zhang Y, Xu J, Shen P (2005) Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci USA 102:13289–13294
Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I et al (2004) Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci 24:6507–6514
Kerr M, Davies SA, Dow JA (2004) Cell-specific manipulation of second messengers; a toolbox for integrative physiology in Drosophila. Curr Biol 14:1468–1474
Thamm M, Balfanz S, Scheiner R, Baumann A, Blenau W (2010) Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell Mol Life Sci 67:2467–2479
Service FJ, O’Brien PC, Rizza RA (1987) Measurements of glucose control. Diabetes Care 10:225–237
Cognigni P, Bailey AP, Miguel-Aliaga I (2011) Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab 13:92–104
Troppmann B, Balfanz S, Baumann A, Blenau W (2010) Inverse agonist and neutral antagonist actions of synthetic compounds at an insect 5-HT1 receptor. British J Pharmacol 159:1450–1462
Agrawal N, Padmanabhan N, Hasan G (2009) Inositol 1, 4, 5- trisphosphate receptor function in Drosophila insulin producing cells. PLoS ONE 4:e6652
Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC et al (1996) WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic neuronal activity in behaving cats: comparison with (S)-WAY-100135. J Pharmacol Exp Ther 278:752–762
Walker GA, Lithgow GJ (2003) Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2:131–139
Mattaliano MD, Montana ES, Parisky KM, Littleton JT, Griffith LC (2007) The Drosophila ARC homolog regulates behavioral responses to starvation. Mol Cell Neurosci 36:211–221
Corl AB, Rodan AR, Heberlein U (2005) Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster. Nat Neurosci 8:18–19
Walkiewicz MA, Stern M (2009) Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS ONE 4:e5072
Nässel DR (1988) Serotonin and serotonin-immunoreactive neurons in the nervous system of insects. Prog Neurobiol 30:1–85
Melcher C, Pankratz MJ (2005) Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 3:e305
Bader R, Colomb J, Pankratz B, Schrock A, Stocker RF et al (2007) Genetic dissection of neural circuit anatomy underlying feeding behavior in Drosophila: distinct classes of hugin-expressing neurons. J Comp Neurol 502:848–856
Wu Q, Wen T, Lee G, Park JH, Cai HN et al (2003) Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron 39:147–161
Miyazaki T, Ito K (2010) Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. J Comp Neurol 518:4147–4181
Campos AR, Lee KJ, Steller H (1995) Establishment of neuronal connectivity during development of the Drosophila larval visual system. J Neurobiol 28:313–329
Sawin-McCormack EP, Sokolowski MB, Campos AR (1995) Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J Neurogenet 10:119–135
Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J et al (2009) Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol 7:e1000229
Adeghate E, Ponery AS, Pallot DJ, Singh J (2001) Distribution of vasoactive intestinal polypeptide, neuropeptide-Y and substance P and their effects on insulin secretion from the in vitro pancreas of normal and diabetic rats. Peptides 22:99–107
Adeghate E, Ponery AS (2002) GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1–6
Mezler M, Muller T, Raming K (2001) Cloning and functional expression of GABA(B) receptors from Drosophila. Eur J Neurosci 13:477–486
Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H et al (1998) Human gamma-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc Natl Acad Sci USA 95:14991–14996
Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84:835–867
Hamasaka Y, Wegener C, Nässel DR (2005) GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J Neurobiol 65:225–240
Kolaj M, Bai D, Renaud LP (2004) GABAB receptor modulation of rapid inhibitory and excitatory neurotransmission from subfornical organ and other afferents to median preoptic nucleus neurons. J Neurophysiol 92:111–122
Acknowledgments
We thank the persons and organizations listed in “Materials and methods” for flies and reagents. This study was supported by the Swedish Research Council (VR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2011_789_MOESM1_ESM.tif
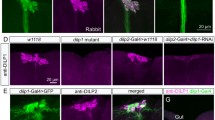
S Fig. 1 The 5-TH1B and 5-HT7 receptors appear not to be expressed in IPCs. Antiserum to DILP2 (magenta) was combined with 5-HT 1B and 5-HT 7 -Gal4 driven GFP (green) in adult and larval brains of Drosophila (all images are projections of several optic sections). a. No colocalization of markers is seen in IPCs in the adult brain. Arrow points at a receptor expressing cell body distinct from the IPCs. In ai and aii the separate channels are shown. b. Also in the larval brain there is no colocalization of markers. c. In the adult brain the 5-HT7 is expressed mainly in neurons of the ellipsoid body (EB), including the lateral triangle (LTR), and not in the IPCs. d. The larval IPCs also do not express 5-HT7, although some adjacent cell bodies do so. (TIFF 6995 kb)
18_2011_789_MOESM2_ESM.tif
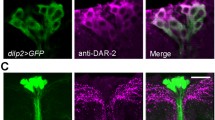
S. Fig. 2 The 5-HT1A receptor expression pattern closely resembles that of serotonergic branches. To reveal the relations between the 5-HT1A receptor and neurons processes releasing the ligand, we applied a monoclonal antibody to serotonin (magenta) and 5-HT 1A -Gal4 driven GFP (green). a – c. In the adult brain the match between the receptor and serotonin-immunoreactive processes is close. Note that the 5-HT1A expression may also include non-dendritic portions of the neurons where no receptor protein is located (accounting for part of the mismatch). Especially in the pars intercerebralis (PI) and ellipsoid body (EB) the matching patterns are seen. d. Expression of 5-HT1A (green) and serotonin (magenta) in the larval ventral nerve cord (total projection). Processses from the numerous 5-HT1A expressing neurons superimpose the serotonergic ones in the two columns of synaptic neuropil. The arrow indicates anterior (a) and posterior (p). e - f. Single channels showing 5-HT 1A -Gal4 driven GFP and serotonin-immunolabeling. (TIFF 8746 kb)
18_2011_789_MOESM3_ESM.tif
S Fig. 3 A different Dilp2 -Gal4 line to drive 5-HT 1A knockdown also renders flies more sensitive to starvation. Another Dilp2-Gal4 line (insertion on 3rd chromosome; [18]) was used for driving 5-HT1A-RNAi in IPCs as a control to establish that the effects seen are not caused by position of insert. As with the other Dilp2-Gal4 driver we observe that 5-HT1A-RNAi in IPCs render flies more sensitive to starvation (p<0.0001 to both controls, Log rank test, Mantel-Cox; n= 180 for each genotype; experiment in three replicates). (TIFF 243 kb)
18_2011_789_MOESM4_ESM.tif
S Fig. 4 Tests of RNAi for 5-HT 1B and 5-HT 7 receptors in IPCs indicate no effect on sensitivity to starvation. Although we have no evidence for expression of 5-HT1B and 5-HT7 receptors in IPCs of Drosophila we tested driving RNAi for the two receptor with the Dilp2-Gal4 line and exposed the different fly crosses to starvation. a. Attempted knockdown of 5-HT1B in IPCs [Dilp2-5-HT1BRi(V)] did not result in a change in survival at starvation compared to controls (p=0.8140 and p=0.4046 to the two controls, p=0.5431 between controls; n= 142-164 for the three genotypes, experiment in three replicates). This was a UAS-5-HT1BRNAi line from VDRC. b. Attempted knockdown of 5-HT7 in IPCs (Dilp2-5-HT7Ri) did not result in a change in survival at starvation compared to controls. (TIFF 2268 kb)
18_2011_789_MOESM5_ESM.tif
S Fig. 5 Knockdown of 5-HT 1A in IPCs or globally does not affect growth. Adult flies of the different genotypes were weighed at the age of 4 – 6 d to estimate growth. a. Knockdown of 5-HT1A in IPCs by Dilp2-Gal4 driven RNAi (Dilp2-5-HT1ARi) does not produce a noticable growth phenotype in male flies (ns, p=0.105 and *** p<0.001; one-way ANOVA, Tukey’s comparison; n = 120 for each genotype, experiment in three replicates). b. Weights of wild type (w1118) and 5-HT1A mutant flies (M, males and F, females) also do not differ (ns, p=0.07 for male mutant to wild type, and p=0.162 for female mutant to control; one-way ANOVA; n= 120 for each genotype and sex). (TIFF 344 kb)
Rights and permissions
About this article
Cite this article
Luo, J., Becnel, J., Nichols, C.D. et al. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor. Cell. Mol. Life Sci. 69, 471–484 (2012). https://doi.org/10.1007/s00018-011-0789-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0789-0