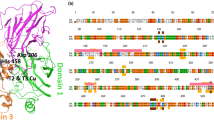
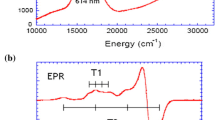
Abstract
Laccases are phenol oxidases that belong to the family of multi-copper oxidases and the superfamily of cupredoxins. A number of potential industrial applications for laccases have led to intensive structure-function studies and an increased amount of crystal structures has been solved. The objective of this review is to summarize and analyze available crystal structures of laccases. The experimental crystallographic data are now easily available from the websites and electron density maps can be used for the interpretation of the structural models. The crystal structures can give valuable insights into the functional mechanisms and may serve as the basis for the development of laccases for industrial applications.






Similar content being viewed by others
References
Ducros V, Brzozowski AM, Wilson KS, Brown SH, Ostergaard P, Schneider P, Yaver DS, Pedersen AH, Davies GJ (1998) Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol 5:310–316
Hakulinen N, Kiiskinen L-L, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J (2002) Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear site. Nat Struct Biol 9:601–605
Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C (2002) Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry 41:7325–7333
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem 277:37663–37669
Enquita FJ, Martis L, Henriques AO, Carrondo MA (2003) Crystal structure of a bacterial endospore coat component. J Biol Chem 278:19416–19425
Kleywegt GJ, Harris MR, Taylor TC, Wählby A, Jones A (2004) The Uppsala electron-density Server. Acta Crystallogr D60:2240–2249
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D 60:2126–2132
Pozharski E, Weichenberger CX, Rupp B (2013) Techniques, tools and best practices for ligand electron-density analysis and results from their application to deposited crystal structures. Acta Cryst D 69:150–167
Murphy LM, Strange RW, Karlsson BG, Lundberg LG, Pascher T, Reinhammar B, Hasnain SS (1993) Structural characterization of azurin from Pseudomonas aeruginosa and some of its methionine-121 mutants. Biochemistry 32:1965–1975
Colman PM, Freeman HC, Guss JM, Murata M, Noms VA, Ramshaw JAM, Venkatappa MP (1978) X-ray crystal structure analysis of plastocyanin at 2.7 Å resolution. Nature 272:319–324
Adman ET, Stenkamp RE, Sieker LC, Jensen LH (1978) A crystallographic model for azurin at 3 Å resolution. J Mol Biol 123:35–47
Murphy MEP, Turley S, Adman ET (1997) Structure of nitrite bound to copper-containing nitrite reductase from Alcaligenes faecalis. J Biol Chem 272:28455–28460
Zaitseva I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A, Lindley P (1996) The X-ray structure of human serum ceruloplasmin at 3.1 Å: nature of the copper centres. J Biol Inorg Chem 1:15–23
Nakamura K, Kawabata T, Yura K, Go N Novel types of two-domain multi-copper oxidases: possible missing links in the evolution. Febs Lett. 553, 239–244
Skálová T, Dohnálek J, Østergaard LH, Østergaard PR, Kolenko P, Dusková J, Stepánková A, Hasek J (2009) The structure of the small laccase from Streptomyces coelicolor reveals a link between laccases and nitrite reductases. J Mol Biol 385:1165–1178
Lawton TJ, Sayavedra-Soto LA, Arp DJ, Rozenweig AC (2009) Crystal structure of a two-domain multicopper oxidase. J Biol Chem 284:10174–10180
Komori H, Miyazaki K, Higuchi Y (2009) X-ray structure of a two-domain type laccase: a missing link in the evolution of multi-copper proteins. FEBS Lett 583:1189–1195
Lyashenko AV, Bento I, Zaitsev VN, Zhukhlistova NE, Zhukova YN, Gabdoulkhakov AG, Morgunova EY, Voelter W, Kachalova GS, Stepanova EV, Koroleva OV, Lamzin VS, Tishkov VI, Betzel C, Lindley PF, Mikhailov AM (2006) X-ray structural studies of the fungal laccase from Cerrena maxima. J Biol Inorg Chem 11:963–973
De la Mora E, Lovett JE, Blanford CF, Garman EF, Valderrama B, Rudino-Pinera E (2012) Structural changes caused by radiation-induced reduction and radiolysis: the effect of X-ray absorbed dose in a fungal multicopper oxdase. Acta Cryst D 78:564–577
Lyashenko AV, Zhukova YN, Zhukhlistova NE, Zaitsev VN, Stepanova EV (2006) Three-dimensional structure of laccase from Coriolus zonatus at 2.6 Å resolution. Crystallogr Rep 51:817–823
Ferraroni M, Myasoedova NM, Schmatchenko V, Leontievsky AA, Golovleva LA, Scozzafava A, Briganti F (2007) Crystal structure of a blue laccase from Lentinus tigrinus: evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Struct Biol 7:60
Antorini M, Herpöel-Gimbert I, Choinowski T, Sigolloit J-C, Asther M, Winterhalter K, Piontek K (2002) Purification, crystallisation and X-ray diffraction study of fully functional laccases from two ligninolytic fungi. Biochim Biophys Acta 1594:109–114
Garavaglia S, Cambria MT, Miglio M, Ragusa S, Iacobazzi V, Palmieri F, D’Ambrosio C, Scaloni A, Rizzi M (2004) The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J Mol Biol 342:1519–1531
Ferraroni M, Matera I, Chernykh A, Kolomytseva M, Golovleva LA, Scozzafava A, Briganti F (2012) Reaction intermediates and redox state changes in a blue laccase from Steccherinum ochraceum observed by crystallographic high/low X-ray dose experiments. J Inorg Biochem 11:203–209
Kallio JP, Gasparetti C, Andberg M, Boer H, Koivula A, Kruus K, Rouvinen J, Hakulinen N (2011) Crystal structure of an ascomycete fungal laccase from Thielavia arenaria: common structural features of asco-laccases. FEBS J 278:2283–2295
Polyakov KM, Fedorova TV, Stepanova EV, Cherkashin EA, Kurzeev SA, Strokopytov BV, Lamzin VS, Koroleva OV (2009) Structure of native laccase from Trametes hirsuta at 1.8 Å resolution. Acta Cryst. D65:611–617
Matera I, Gullotto A, Tilli S, Ferraroni M, Scozzafava A, Briganti F (2008) Crystal structure of the blue multicopper oxidase from the whiterot fungus Trametes trogii complexed with p-toluate. Inorg Chim Acta 361:4129–4137
Roberts SA, Wildner GF, Grass G, Weichsel A, Ambrus A, Rensing C, Montfort WR (2003) A labile regulatory copper ion lies near the T1 copper site in the multicopper oxidase CueO. J Biol Chem 278:31958–31963
Silva CS, Durao P, Fillat A, Lindley PF, Martins L, Bento I (2012) Crystal structure of the multicopper oxidase from the pathogenic bacterium Campylobacter jejuni CGUG11284: characterization of a metallo-oxidase. Metallomics 4:37–47
Taylor A, Stoj CS, Ziegler L, Kosman DJ, Hart J (2005) The copper–iron connection in biology: structure of a metallo-oxidase Fet3p. PNAS 102:15459–15464
Messerschmidt A, Ladenstein R, Huber R (1992) Refined crystal structure of ascorbate oxidase at 1.9 Å resolution. J Mol Biol 224:179–205
Izutani K, Toyoda M, Sagara K, Takahashi N, Sato A, Kamitaka Y, Tsujimura S, Nakanishi Y, Yamaguchi S, Kanob K, Mikamia B (2010) X-ray analysis of bilirubin oxidase from Myrothecium verrucaria at 2.3 Å resolution using a twinned crystal. Acta Cryst F 66(7):765–770
Cracknell JA, McNamara TP, Lowe ED, Blanford CF (2011) Bilirubin oxidase from Myrothecium verrucaria: x-ray determination of the complete crystal structure and a rational surface modification for enhanced electrocatalytic O2 reduction. Dalton Trans 40:765–770
Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Gronan JE, Gerlt JA (2014) Roles of small laccases from Streptomyces in lignin degradation. Biochemistry 53:4047–4058
Gunne M, Höppner A Hagedoorn P-L, Urlacher VB (2014) Structural and redox properties of the small laccase Ssl1 from Streptomyces sviceus FEBS J 281:4307–4311
Kallio JP, Auer S, Jänis J, Andberg M, Kruus K, Rouvinen J, Koivula A, Hakulinen N (2009) Structure-function studies of a Melanocarpus albomyces laccase suggest a pathway for oxidation of phenolic compounds. J Mol Biol 392:895–909
Enguita FJ, Marçal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA (2004) Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J Biol Chem 279:23472–23476
Bento I, Martins LO, Lopes GG, Carrondo MA, Lindley PF (2005) Dioxygen reduction by multi-copper oxidases; a structural perspective. Dalton Trans 7:3507–3513
Hakulinen N, Kruus K, Koivula A, Rouvinen J (2006) A crystallographic and spectroscopic study on the effect of X-ray radiation on the crystal structure of Melanocarpus albomyces laccase. Biochem Biophys Res Commun 350:929–934
Andberg M, Hakulinen N, Auer S, Saloheimo M, Koivula A, Rouvinen J, Kruus K (2009) Essential role of the C-terminus in Melanocarpus albomyces laccase for enzyme production, catalytic properties and structure. FEBS J 276:6285–6300
Durao P, Chen CS, Silva CS, Soares CM, Pereira MM, Todorovic S, Hildebrandt P, Bento I, Lindley PF, Martins LO (2008) Proximal Mutations at the type 1 copper site of cota laccase: spectroscopic, redox, kinetic and structural characterization of I494a and L386a mutants. Biochem J 412:339–346
Chen Z, Durao P, Silva CS, Pereira MM, Todorovic S, Hildebrandt P, Bento I, Lindley PF, Martins LO (2010) The role of Glu498 in the dioxygen reactivity of cota-laccase from Bacillus Subtilis. Dalton Trans 39:2875–2882
Silva CS, Damas JM, Chen Z, Brissos V, Martins LO, Soares CM, Lindley PF, Bento I (2012) The role of Asp116 in the reductive cleavage of dioxygen to water in cota laccase: assistance during the proton transfer mechanism. Acta Cryst D 68:186–193
Gupta A, Nederlof I, Sottini S, Tepper AWJW, Groenen EJJ, Thomassen EAJ, Canters GW (2012) Involvement of Tyr108 in the enzyme mechanism of the small laccase from Streptomyces coelicolor. J Am Chem Soc 134:18213–18216
Kallio JP, Rouvinen J, Kruus K, Hakulinen N (2011) Probing the dioxygen route in Melanocarpus albomyces laccase with pressurized xenon gas. Biochemistry 50:4396–4398
Quintanar L, Stoj C, Wang TP, Kosman DJ, Solomon EI (2005) Role of spartate 94 in the decay of the peroxide intermediate in the multicopper oxidase Fet3p. Biochemistry 44:6081–6091
Ferraroni M, Matera I, Chernykh A, Kolomytseva M, Golovleva LA, Scozzafava A, Briganti F (2012) Reaction intermediates and redox state changes in a blue laccase from Steccherinum ochraceum observed by crystallographic high/low X-ray dose experiments. J Inorg Biochem 111:203–209
De la Mora E, Lovett JE, Blanford C, Gramna EF, Valderrama B, Rudino-Pinera E (2012) Structural changes caused by radiation-induced reduction and radiolysis: the effect of X-ray absorbed dose in a fungal multicopper oxidase. Acta Cryst D 68:564–577
Komori H, Sugiyama R, Kataoka K, Miyazaki K, Higuchi Y, Sakurai T (2014) New insights into the catalytic-site structure of multicopper oxidases. Acta Cryst D70:772–779
Acknowledgements
The work was supported by the Academy of Finland (Project 256937 and 263931).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hakulinen, N., Rouvinen, J. Three-dimensional structures of laccases. Cell. Mol. Life Sci. 72, 857–868 (2015). https://doi.org/10.1007/s00018-014-1827-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1827-5