Abstract
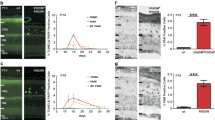
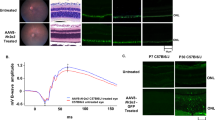
The enzyme poly-ADP-ribose-polymerase (PARP) has important roles for many forms of DNA repair and it also participates in transcription, chromatin remodeling and cell death signaling. Currently, some PARP inhibitors are approved for cancer therapy, by means of canceling DNA repair processes and cell division. Drug repurposing is a new and attractive aspect of therapy development that could offer low-cost and accelerated establishment of new treatment options. Excessive PARP activity is also involved in neurodegenerative diseases including the currently untreatable and blinding retinitis pigmentosa group of inherited retinal photoreceptor degenerations. Hence, repurposing of known PARP inhibitors for patients with non-oncological diseases might provide a facilitated route for a novel retinitis pigmentosa therapy. Here, we demonstrate and compare the efficacy of two different PARP inhibitors, BMN-673 and 3-aminobenzamide, by using a well-established retinitis pigmentosa model, the rd1 mouse. Moreover, the mechanistic aspects of the PARP inhibitor-induced protection were also investigated in the present study. Our results showed that rd1 rod photoreceptor cell death was decreased by about 25–40% together with the application of these two PARP inhibitors. The wealth of human clinical data available for BMN-673 highlights a strong potential for a rapid clinical translation into novel retinitis pigmentosa treatments. Remarkably, we have found that the efficacy of 3 aminobenzamide was able to decrease PARylation at the nanomolar level. Our data also provide a link between PARP activity with the Wnt/β-catenin pathway and the major intracellular antioxidant concentrations behind the PARP-dependent retinal degeneration. In addition, molecular modeling studies were integrated with experimental studies for better understanding of the role of PARP1 inhibitors in retinal degeneration.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information file.
Abbreviations
- ADPRT:
-
ADP-ribosyltransferase
- ART:
-
ADP-ribosyltransferase
- AIF:
-
Apoptosis-inducing factor
- DAPI:
-
4′,6-diamidino-2-phenylindole
- EDTA:
-
Ethylenediaminetetraacetic acid
- GCL:
-
Ganglion cell layer
- GSH:
-
Glutathion
- GFAP:
-
Glial fibrillary acidic protein
- GSK:
-
Glycogen synthase kinase
- INL:
-
Inner nuclear layer
- min:
-
Minutes
- ODU:
-
Optical density units
- ONL:
-
Outer nuclear layer
- PDE6:
-
Phosphodiesterase-6
- PARP:
-
Poly-ADP-ribose-polymerase
- P:
-
Postnatal day
- RP:
-
Retinitis pigmentosa
- rd1:
-
Retinal degeneration 1
- RPE:
-
Retinal pigment epithelium
- SEM:
-
Standard error of the mean
- s:
-
Seconds
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- TMU:
-
N-(4-methoxybenzyl)-N′-(5-nitro-1,23-thiazol-2-yl)urea
- wt:
-
Wild-type
References
Parmeggiani F (2011) Clinics, epidemiology and genetics of retinitis pigmentosa. Curr Genom 12:236–237. https://doi.org/10.2174/138920211795860080
Scholl HP, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel JA (2016) Emerging therapies for inherited retinal degeneration. Sci Transl Med 8:368rv6. https://doi.org/10.1126/scitranslmed.aaf2838
Hassa PO, Hottiger MO (2008) The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci 13(3046–3082):2909
Martire S, Mosca L, d’Erme M (2015) PARP-1 involvement in neurodegeneration: a focus on Alzheimer’s and Parkinson’s diseases. Mech Ageing Dev 146–148:53–64. https://doi.org/10.1016/j.mad.2015.04.001
Berger NA, Besson VC, Boulares AH, Burkle A, Chiarugi A, Clark RS, Curtin NJ, Cuzzocrea S, Dawson TM, Dawson VL, Hasko G, Liaudet L, Moroni F, Pacher P, Radermacher P, Salzman AL, Snyder SH, Soriano FG, Strosznajder RP, Sumegi B, Swanson RA, Szabo C (2018) Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol 175:192–222. https://doi.org/10.1111/bph.13748
Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, Lee BD, Kang HC, Kim D, Tessarollo L, Dawson VL, Dawson TM (2013) Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci 16:1392–1400. https://doi.org/10.1038/nn.3500
Weaver AN, Yang ES (2013) Beyond DNA repair: additional functions of PARP-1 in cancer. Front Oncol 3:290. https://doi.org/10.3389/fonc.2013.00290
Cipriani G, Rapizzi E, Vannacci A, Rizzuto R, Moroni F, Chiarugi A (2005) Nuclear poly(ADP-ribose) polymerase-1 rapidly triggers mitochondrial dysfunction. J Biol Chem 280:17227–17234. https://doi.org/10.1074/jbc.M414526200
Hong SJ, Dawson TM, Dawson VL (2004) Nuclear and mitochondrial conversations in cell death: pARP-1 and AIF signaling. Trends Pharmacol Sci 25:259–264. https://doi.org/10.1016/j.tips.2004.03.005
Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297:259–263. https://doi.org/10.1126/science.1072221
Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K (2004) Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab 24:458–466. https://doi.org/10.1097/00004647-200404000-00011
Arango-Gonzalez B, Trifunovic D, Sahaboglu A, Kranz K, Michalakis S, Farinelli P, Koch S, Koch F, Cottet S, Janssen Bienhold U, Dedek K, Biel M, Zrenner E, Euler T, Ekstrom PAR, Ueffing M, Paquet-Durand F (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9:e112142
Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ, van Veen T, Zrenner E, Ekstrom P, Paquet-Durand F (2008) Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol Neurobiol 38:253–269. https://doi.org/10.1007/s12035-008-8045-9
Sahaboglu A, Tanimoto N, Kaur J, Sancho-Pelluz J, Huber G, Fahl E, Arango-Gonzalez B, Zrenner E, Ekstrom P, Lowenheim H, Seeliger M, Paquet-Durand F (2010) PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. PLoS One 5:e15495
Sahaboglu A, Barth M, Secer E, Amo EM, Urtti A, Arsenijevic Y, Zrenner E, Paquet-Durand F (2016) Olaparib significantly delays photoreceptor loss in a model for hereditary retinal degeneration. Sci Rep 6:39537. https://doi.org/10.1038/srep39537
Sahaboglu A, Sharif A, Feng L, Secer E, Zrenner E, Paquet-Durand F (2017) Temporal progression of PARP activity in the Prph2 mutant rd2 mouse: neuroprotective effects of the PARP inhibitor PJ34. PLoS One 12:e0181374. https://doi.org/10.1371/journal.pone.0181374
Bitler BG, Watson ZL, Wheeler LJ, Behbakht K (2017) PARP inhibitors: clinical utility and possibilities of overcoming resistance. Gynecol Oncol 147:695–704. https://doi.org/10.1016/j.ygyno.2017.
Lim JSJ, Tan DSP (2017) Understanding resistance mechanisms and expanding the therapeutic utility of PARP inhibitors. Cancers (Basel). https://doi.org/10.3390/cancers9080109
Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB (1990) Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 347:677–680. https://doi.org/10.1038/347677a0
Sanyal S, Bal AK (1973) Comparative light and electron microscopic study of retinal histogenesis in normal and rd mutant mice. Z Anat Entwicklungsgesch 142:219–238
Caffe AR, Ahuja P, Holmqvist B, Azadi S, Forsell J, Holmqvist I, Soderpalm AK, van Veen T (2001) Mouse retina explants after long-term culture in serum free medium. J Chem Neuroanat 22:263–273
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW (1980) High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem 106:55–62. https://doi.org/10.1016/0003-2697(80)90118-9
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267:727–748. https://doi.org/10.1006/jmbi.1996.0897
Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234. https://doi.org/10.1007/s10822-013-9644-8
UniProt Consortium (2015) UniProt: a hub for protein information. Nucleic Acids Res 43:D204–D212. https://doi.org/10.1093/nar/gku989
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Bienert S, Waterhouse A, de Beer TA, Tauriello G, Studer G, Bordoli L, Schwede T (2017) The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res 45:D313–D319. https://doi.org/10.1093/nar/gkw1132
Guex N, Peitsch MC, Schwede T (2009) Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30(Suppl 1):S162–S173. https://doi.org/10.1002/elps.200900140
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350. https://doi.org/10.1093/bioinformatics/btq662
Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG (2012) ZINC: a free tool to discover chemistry for biology. J Chem Inf Model 52:1757–1768. https://doi.org/10.1021/ci3001277
Irwin JJ, Shoichet BK (2005) ZINC—a free database of commercially available compounds for virtual screening. J Chem Inf Model 45:177–182. https://doi.org/10.1021/ci049714
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J, Yu B, Zhang J, Bryant SH (2016) PubChem substance and compound databases. Nucleic Acid Res 44:D1202–D1213. https://doi.org/10.1093/nar/gkv951
Paquet-Durand F, Silva J, Talukdar T, Johnson LE, Azadi S, van Veen T, Ueffing M, Hauck SM, Ekstrom PA (2007) Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J Neurosci 27:10311–10319
Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, Wang B, Lord CJ, Post LE, Ashworth A (2013) BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res 19:5003–5015. https://doi.org/10.1158/1078-0432.CCR-13-1391
Jones J, Patel BN, Skidmore CJ (1988) Benzamides can stimulate as well as inhibit the activity of nuclear ADP-ribosyltransferase. Carcinogenesis 9:2023–2026. https://doi.org/10.1093/carcin/9.11.2023
Rankin PW, Jacobson EL, Benjamin RC, Moss J, Jacobson MK (1989) Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem 264:4312–4317
Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG (2010) PARP inhibition: parp1 and beyond. Nat Rev Cancer 10:293–301. https://doi.org/10.1038/nrc2812
Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM (2018) WNT signaling in cardiac and vascular disease. Pharmacol Rev 70:68–141. https://doi.org/10.1124/pr.117.013896
Wu D, Pan W (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35:161–168
Yang E, Tacchelly-Benites O, Wang Z, Randall MP, Tian A, Benchabane H, Freemantle S, Pikielny C, Tolwinski NS, Lee E, Ahmed Y (2016) Wnt pathway activation by ADP-ribosylation. Nat Commun 7:11430. https://doi.org/10.1038/ncomms11430
Antolin AA, Mestres J (2014) Linking off-target kinase pharmacology to the differential cellular effects observed among PARP inhibitors. Oncotarget 5:3023–3028. https://doi.org/10.18632/oncotarget.1814
Mariotti L, Pollock K, Guettler S (2017) Regulation of Wnt/beta-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding. Br J Pharmacol 174:4611–4636. https://doi.org/10.1111/bph.14038
Mustafi D, Engel AH, Palczewski K (2009) Structure of cone photoreceptors. Prog Retin Eye Res 28:289–302. https://doi.org/10.1016/j.preteyeres.2009.05.003
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. 267:727–748. https://doi.org/10.1006/jmbi.1996.0897
Bhat R, Xue Y, Berg S, Hellberg S, Ormo M, Nilsson Y, Radesater AC, Jerning E, Markgren PO, Borgegard T, Nylof M, Gimenez-Cassina A, Hernandez F, Lucas JJ, Diaz-Nido J, Avila J (2003) Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem 278:45937–45945. https://doi.org/10.1074/jbc.m306268200
Ye Q, Li M, Zhou Y, Pang T, Xu L, Cao J, Han L, Li Y, Wang W, Gao J, Li J (2013) Synthesis and biological evaluation of 3-benzisoxazolyl-4-indolylmaleimides as potent, selective inhibitors of glycogen synthase kinase-3beta. Molecules 18:5498–5516. https://doi.org/10.3390/molecules18055498
Ekstrom P, Sanyal S, Narfstrom K, Chader GJ, van Veen T (1988) Accumulation of glial fibrillary acidic protein in Muller radial glia during retinal degeneration. Invest Ophthalmol Vis Sci 29:1363–1371
Lewis GP, Fisher SK (2003) Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol 230:263–290
Winkler BS, Giblin FJ (1983) Glutathione oxidation in retina: effects on biochemical and electrical activities. Exp Eye Res 36:287–297
Sanchez-Vallejo V, Benlloch-Navarro S, Trachsel-Moncho L, Lopez-Pedrajas R, Almansa I, Romero FJ, Miranda M (2016) Alterations in glutamate cysteine ligase content in the retina of two retinitis pigmentosa animal models. 96:245–254. https://doi.org/10.1016/j.freeradbiomed.2016.04.195
McCluskey JD, Sava D, Harbison SC, Muro-Cacho CA, Giffe JT, Ping X, Harbison RD (2011) Hepatoprotective effects of select water-soluble PARP inhibitors in a carbon tetrachloride model. Int J Crit Illn Inj Sci 1:97–103. https://doi.org/10.4103/2229-5151.84788
Zakaria EM, El-Bassossy HM, El-Maraghy NN, Ahmed AF, Ali AA (2016) PARP-1 inhibition alleviates diabetic cardiac complications in experimental animals. Eur J Pharmacol 791:444–454. https://doi.org/10.1016/j.ejphar.2016.09.008
Kaur J, Mencl S, Sahaboglu A, Farinelli P, van Veen T, Zrenner E, Ekstrom P, Paquet-Durand F, Arango-Gonzalez B (2011) Calpain and PARP activation during photoreceptor cell death in P23H and S334ter rhodopsin mutant rats. PLoS One 6:e22181. https://doi.org/10.1371/journal.pone.0022181
Mirza MR, Pignata S, Ledermann JA (2018) Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol 29:1366–1376. https://doi.org/10.1093/annonc/mdy174
Cleaver JE, Morgan WF (1987) 3-Aminobenzamide, an inhibitor of poly(ADP-ribose) polymerase, is a stimulator, not an inhibitor, of DNA repair. Exp Cell Res 172:258–264. https://doi.org/10.1016/0014-4827(87)90385-5
Bauer PI, Hakam A, Kun E (1986) Mechanisms of poly(ADP-ribose) polymerase catalysis; mono-ADP-ribosylation of poly(ADP-ribose) polymerase at nanomolar concentrations of NAD. FEBS Lett 195:331–338. https://doi.org/10.1016/0014-5793(86)80188-0
Jiao K, Sahaboglu A, Zrenner E, Ueffing M, Ekstrom PA, Paquet-Durand F (2016) Efficacy of PARP inhibition in Pde6a mutant mouse models for retinitis pigmentosa depends on the quality and composition of individual human mutations. Cell Death Discov 2:16040. https://doi.org/10.1038/cddiscovery.2016.40
Songin M, Jesko H, Czapski G, Adamczyk A, Strosznajder RP (2007) GSK-3beta and oxidative stress in aged brain. Role of poly(ADP- -ribose) polymerase-1. Folia Neuropathol 45:220–229
Kovacs K, Vaczy A, Fekete K, Kovari P, Atlasz T, Reglodi D, Gabriel R, Gallyas F, Sumegi B (2019) PARP inhibitor protects against chronic hypoxia/reoxygenation-induced retinal injury by regulation of MAPKs, HIF1alpha, Nrf2, and NFkappaB. Invest Ophthalmol Vis Sci 60:1478–1490. https://doi.org/10.1167/iovs.18-25936
Patel AK, Surapaneni K, Yi H, Nakamura RE, Karli SZ, Syeda S, Lee T, Hackam AS (2015) Activation of Wnt/beta-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology 91:1–12. https://doi.org/10.1016/j.neuropharm.2014.11.015
Paasche G, Huster D, Reichenbach A (1998) The glutathione content of retinal Muller (glial) cells: the effects of aging and of application of free-radical scavengers. Ophthalmic Res 30:351–360. https://doi.org/10.1159/000055495
Bringmann A, Wiedemann P (2012) Muller glial cells in retinal disease. Ophthalmologica 227:1–19. https://doi.org/10.1159/000328979
Hippert C, Graca AB, Barber AC, West EL, Smith AJ, Ali RR, Pearson RA (2015) Muller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One 10:e0120415. https://doi.org/10.1371/journal.pone.0120415
Huster D, Reichenbach A, Reichelt W (2000) The glutathione content of retinal Muller (glial) cells: effect of pathological conditions. Neurochem Int 36:461–469
Olsen JJ, Pohl SO, Deshmukh A, Visweswaran M, Ward NC, Arfuso F, Agostino M, Dharmarajan A (2017) The role of Wnt signalling in angiogenesis. Clin Biochem Rev 38:131–142
Wang Y, Sang A, Zhu M, Zhang G, Guan H, Ji M, Chen H (2016) Tissue factor induces VEGF expression via activation of the Wnt/beta-catenin signaling pathway in ARPE-19 cells. Mol Vis 22:886–897
Nishiguchi KM, Nakamura M, Kaneko H, Kachi S, Terasaki H (2007) The role of VEGF and VEGFR2/Flk1 in proliferation of retinal progenitor cells in murine retinal degeneration. Invest Ophthalmol Vis Sci 48:4315–4320. https://doi.org/10.1167/iovs.07-0354
Acknowledgements
We thank Per Ekström, Eberhart Zrenner and Wadood Haq for scientific advice and discussions, and Sylvie Bolz and Christine Henes for excellent technical assistance. This work was supported by Deutsche Forschungsgemeinschaft (DFG; SA3040/1-1, DFG; SA3040/3-1), the Charlotte and Tistou Kerstan Foundation (SAH001/2016).
Author information
Authors and Affiliations
Contributions
AS carried out the in vitro retinal explant culture studies, and AS, NS, ES and JAFP carried out the analysis of immunohistology. SD and GK performed the studies on in silico drug analysis and MM carried out the analysis of GSH. MAA and DC performed GSK-alpha activity assay. AS conceived the study, AS, SD and MM participated in the design, analysis, coordination and interpretation of the study and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahaboglu, A., Miranda, M., Canjuga, D. et al. Drug repurposing studies of PARP inhibitors as a new therapy for inherited retinal degeneration. Cell. Mol. Life Sci. 77, 2199–2216 (2020). https://doi.org/10.1007/s00018-019-03283-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03283-2