Abstract
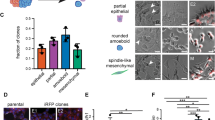
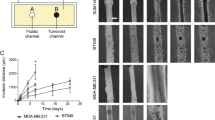
Cancer stem-like cells (CSCs) are a typically small subpopulation of highly tumorigenic cells that can self-renew, differentiate, drive tumor progression, and may mediate drug resistance and metastasis. Metastasis driving CSCs are expected to be highly invasive. To determine the relative invasiveness of CSCs, we isolate distinct subpopulations in the metastatic, MDA-MB-231 breast-cancer cell line, identified by the stem-cell markers aldehyde dehydrogenase (ALDH) and CD44. We determine CSC-subpopulation invasiveness levels using our rapid (2 h) mechanobiology-based assay. Specifically, invasive cells forcefully push and indent the surface of physiological–stiffness synthetic gels to cell-scale depths, where the percentage of indenting cells and their attained depths have previously provided clinically relevant predictions of the metastatic risk in different cancer types. We observe that the small (3.2%) CD44+ALDH+ cell-subpopulation indents more and attains significantly deeper depths (65% indenting to 6 ± 0.3 µm) relative to CD44+ALDH−, CD44−ALDH−, CD44−ALDH+ cells, and the whole-sample control (with 18–44% indenting cells reaching average depths of 4.4–5 µm). The CD44+ALDH+ similarly demonstrates twofold higher migratory capacity in Boyden chambers. The higher invasiveness of CD44+ALDH+ cells reveals their likely role in facilitating disease progression, providing prognostic markers for increased risk of recurrence and metastasis.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CSCs:
-
Cancer stem-like cells
- ALDH:
-
Aldehyde dehydrogenase
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- APS:
-
Ammonium persulfate
- TEMED:
-
Tetramethylethylenediamine
- ANOVA:
-
Analysis of variance
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Joensuu H, Gligorov J (2012) Adjuvant treatments for triple-negative breast cancers. Ann Oncol 23(Suppl 6):vi40–vi45. https://doi.org/10.1093/annonc/mds194
Gal N, Weihs D (2012) Intracellular mechanics and activity of breast cancer cells correlate with metastatic potential. Cell Biochem Biophys 63:199–209
Massalha S, Weihs D (2017) Metastatic breast cancer cells adhere strongly on varying stiffness substrates, initially without adjusting their morphology. Biomech Model Mechanobiol 16:961–970. https://doi.org/10.1007/s10237-016-0864-4
Kristal-Muscal R, Dvir L, Weihs D (2013) Metastatic cancer cells tenaciously indent impenetrable, soft substrates. New J Phys 15:035022. https://doi.org/10.1088/1367-2630/15/3/035022
Merkher Y, Horesh Y, Abramov Z et al (2020) Rapid cancer diagnosis and early prognosis of metastatic risk based on mechanical invasiveness of sampled cells. Ann Biomed Eng 48:2846–2858. https://doi.org/10.1007/s10439-020-02547-4
Dalerba P, Cho RW, Clarke MF (2007) Cancer Stem Cells: Models and Concepts. Annu Rev Med 58:267–284. https://doi.org/10.1146/annurev.med.58.062105.204854
Saeg F, Anbalagan M (2018) Breast cancer stem cells and the challenges of eradication: a review of novel therapies. Stem cell Investig 5:39. https://doi.org/10.21037/sci.2018.10.05
Velasco-Velázquez MA, Popov VM, Lisanti MP, Pestell RG (2011) The role of breast cancer stem cells in metastasis and therapeutic implications. Am J Pathol 179:2–11. https://doi.org/10.1016/j.ajpath.2011.03.005
Barenholz-Cohen T, Merkher Y, Haj J et al (2020) Lung mechanics modifications facilitating metastasis are mediated in part by breast cancer-derived extracellular vesicles. Int J Cancer 147:2924–2933. https://doi.org/10.1002/ijc.33229
de Beça FF, Caetano P, Gerhard R et al (2013) Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol 66:187–191. https://doi.org/10.1136/jclinpath-2012-201169
Li W, Ma H, Zhang J et al (2017) Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep 7:13856. https://doi.org/10.1038/s41598-017-14364-2
Wang H, Wang L, Song Y et al (2017) CD44+/CD24- phenotype predicts a poor prognosis in triple-negative breast cancer. Oncol Lett 14:5890–5898. https://doi.org/10.3892/ol.2017.6959
Al-Hajj M, Wicha MS, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci 100:3983–3988. https://doi.org/10.1073/pnas.0530291100
Hurt EM, Kawasaki BT, Klarmann GJ et al (2008) CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer 98:756–765. https://doi.org/10.1038/sj.bjc.6604242
Li C, Heidt DG, Dalerba P et al (2007) Identification of pancreatic cancer stem cells. Cancer Res 67:1030–1037. https://doi.org/10.1158/0008-5472.CAN-06-2030
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445:106–110. https://doi.org/10.1038/nature05372
Liu R, Wang X, Chen GY et al (2007) The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356:217–226. https://doi.org/10.1056/NEJMoa063994
Meyer MJ, Fleming JM, Ali MA et al (2009) Dynamic regulation of CD24 and the invasive, CD44posCD24negphenotype in breast cancer cell lines. Breast Cancer Res 11:R82. https://doi.org/10.1186/bcr2449
Meyer MJ, Fleming JM, Lin AF et al (2010) CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer Res 70:4624–4633. https://doi.org/10.1158/0008-5472.CAN-09-3619
Vikram R, Chou WC, Hung SC, Shen CY (2020) Tumorigenic and metastatic role of CD44−/low/CD24−/low cells in luminal breast cancer. Cancers (Basel) 12:1239. https://doi.org/10.3390/cancers12051239
Mylona E, Giannopoulou I, Fasomytakis E et al (2008) The clinicopathologic and prognostic significance of CD44+/CD24-/low and CD44-/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol 39:1096–1102. https://doi.org/10.1016/j.humpath.2007.12.003
Ginestier C, Hur MH, Charafe-Jauffret E et al (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567. https://doi.org/10.1016/j.stem.2007.08.014
Prat A, Parker JS, Karginova O et al (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12:R68. https://doi.org/10.1186/bcr2635
Merkher Y, Weihs D (2017) Proximity of metastatic cells enhances their mechanobiological invasiveness. Ann Biomed Eng 45:1399–1406. https://doi.org/10.1007/s10439-017-1814-8
Merkher Y, Alvarez-Elizondo MB, Weihs D (2017) Taxol reduces synergistic, mechanobiological invasiveness of metastatic cells. Converg Sci Phys Oncol 3:044002. https://doi.org/10.1088/2057-1739/aa8c0b
Alvarez-Elizondo MB, Merkher Y, Shleifer G et al (2021) Actin as a target to reduce cell invasiveness in initial stages of metastasis. Ann Biomed Eng 49:1342–1352. https://doi.org/10.1007/s10439-020-02679-7
Weihs D, Merkher Y (2019) A device and method for determining cell indention activity. Patent pending
Kortam S, Merkher Y, Kramer A et al (2021) Rapid, quantitative prediction of tumor invasiveness in non-melanoma skin cancers using mechanobiology-based assay. Biomech Model Mechanobiol 20:1767–1774. https://doi.org/10.1007/s10237-021-01475-z
Alvarez-Elizondo MB, Weihs D (2017) Cell-gel mechanical interactions as an approach to rapidly and quantitatively reveal invasive subpopulations of metastatic cancer cells. Tissue Eng Part C Methods 23:180–187. https://doi.org/10.1089/ten.TEC.2016.0424
Dvir L, Nissim R, Alvarez-Elizondo MB, Weihs D (2015) Quantitative measures to reveal coordinated cytoskeleton-nucleus reorganization during in vitro invasion of cancer cells. New J Phys 17:043010. https://doi.org/10.1088/1367-2630/17/4/043010
Alvarez-Elizondo MB, Rozen R, Weihs D (2018) Mechanobiology of metastatic cancer. In: Mechanobiology in Health and Disease. Academic Press, pp 449–494
Ben-David Y, Weihs D (2021) Modeling force application configurations and morphologies required for cancer cell invasion. Biomech Model Mechanobiol 20:1187–1194. https://doi.org/10.1007/s10237-021-01441-9
Fillmore CM, Kuperwasser C (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 10:R25. https://doi.org/10.1186/bcr1982
Alvarez-Elizondo MB, Li CW, Marom A et al (2020) Micropatterned topographies reveal measurable differences between cancer and benign cells. Med Eng Phys 75:5–12. https://doi.org/10.1016/j.medengphy.2019.11.004
Suman S, Das TP, Damodaran C (2013) Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br J Cancer 109:2587–2596. https://doi.org/10.1038/bjc.2013.642
Buxboim A, Rajagopal K, Brown AEX, Discher DE (2010) How deeply cells feel: methods for thin gels. J Phys-Condensed Matter 22:194116. https://doi.org/10.1088/0953-8984/22/19/194116
Sen S, Engler AJ, Discher DE (2009) Matrix strains induced by cells: computing how far cells can feel. Cell Mol Bioeng 2:39–48. https://doi.org/10.1007/s12195-009-0052-z
Ricardo S, Vieira AF, Gerhard R et al (2011) Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol 64:937–946. https://doi.org/10.1136/jcp.2011.090456
Sheridan C, Kishimoto H, Fuchs RK et al (2006) CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 8:R59. https://doi.org/10.1186/bcr1610
Idowu MO, Kmieciak M, Dumur C et al (2012) CD44 +/CD24 -/low cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol 43:364–373. https://doi.org/10.1016/j.humpath.2011.05.005
Ahmed MAH, Aleskandarany MA, Rakha EA et al (2012) A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat 133:979–995. https://doi.org/10.1007/s10549-011-1865-8
Sun Q, Wang Y, Officer A et al (2022) Stem-like breast cancer cells in the activated state resist genetic stress via TGFBI-ZEB1. npj Breast Cancer 8:5. https://doi.org/10.1038/s41523-021-00375-w
Croker AK, Goodale D, Chu J et al (2009) High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 13:2236–2252. https://doi.org/10.1111/j.1582-4934.2008.00455.x
Wagoner Johnson A, Harley BA (2011) Mechanobiology of cell-cell and cell-matrix interactions. Springer, New York
Mohammadalipour A, Burdick MM, Tees DFJ (2018) Deformability of breast cancer cells in correlation with surface markers and cell rolling. FASEB J 32:1806–1817. https://doi.org/10.1096/fj.201700762R
Lv J, Liu Y, Cheng F et al (2021) Cell softness regulates tumorigenicity and stemness of cancer cells. EMBO J 40:e106123. https://doi.org/10.15252/EMBJ.2020106123
Nogami T, Shien T, Tanaka T et al (2014) Expression of ALDH1 in axillary lymph node metastases is a prognostic factor of poor clinical outcome in breast cancer patients with 1–3 lymph node metastases. Breast Cancer 21:58–65. https://doi.org/10.1007/s12282-012-0350-5
Wiener GI, Kadosh D, Weihs D (2021) Mechanical interactions of invasive cancer cells through their substrate evolve from additive to synergistic. J Biomech 129:110759
Jayatilaka H, Tyle P, Chen JJ et al (2017) Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat Commun 8:15584. https://doi.org/10.1038/ncomms15584
Lee YH, Morrison BL, Bottaro DP (2014) Synergistic signaling of tumor cell invasiveness by hepatocyte growth factor and hypoxia. J Biol Chem 289:20448–20461. https://doi.org/10.1074/jbc.M114.580597
Guo J, Liu C, Zhou X et al (2017) Conditioned medium from malignant breast cancer cells induces an EMT-like phenotype and an altered N-glycan profile in normal epithelial MCF10A cells. Int J Mol Sci 18:1528. https://doi.org/10.3390/ijms18081528
Acknowledgements
The work was partially supported by the Israeli Ministry of Science and Technology (MOST) Medical Devices Program (Grant no. 3-17427), by the Samuel H. Born Fund for Biomedical Research, and by the Russel Berrie Nano Institute at the Technion-IIT.
Funding
The work was partially supported by the Israeli Ministry of Science and Technology (MOST) Medical Devices Program (Grant no. 3–17427), the Samuel H. Born Fund for Biomedical Research, and by the Russel Berrie Nano Institute at the Technion-IIT.
Author information
Authors and Affiliations
Contributions
MBA-E and DW contributed to the study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by MBA-E and DW.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alvarez-Elizondo, M.B., Weihs, D. Breast cancer stem cells: mechanobiology reveals highly invasive cancer cell subpopulations. Cell. Mol. Life Sci. 79, 134 (2022). https://doi.org/10.1007/s00018-022-04181-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04181-w