Abstract
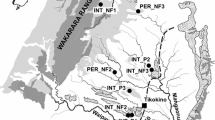
Ecosystem functioning is influenced by the flow of nutrients, detritus, and organisms. Variation in these flows, like that found in temporary ecosystems, affects temporal and spatial patterns of community diversity and secondary production. We evaluated the influence of hydroperiod and ecosystem size on the bi-directional flow of subsidies from intermittent ponds and surrounding forests by quantifying litter deposition and the abundance and biomass of emerging insects and amphibians. In addition, we assessed whether amphibian and insect diversity influenced the magnitude of cross-habitat resource flux. We found substantial spatial and temporal variation in the magnitude, composition, and timing of cross-habitat resource subsidies. Overall, deposition into ponds far exceeded biomass exported via insect and amphibian emergence. We found a negative association between resource flux and the diversity of amphibians and insects. Different species groups contributed to flux patterns unequally, with insects having higher diversity but lower flux compared to amphibians. Organismal flux varied among ponds with amphibians having the highest flux in the shortest hydroperiod pond and insect flux was highest from an intermediate hydroperiod pond. This work reveals how variation in pond size and permanence affects species diversity and ecosystem flows. Species composition played a major role in flux differences across ponds. Further, given the general lack of research and conservation prioritization of temporary ponds, uncovering how these ponds contribute to cross-habitat linkages is necessary to develop fully integrated management strategies.






Similar content being viewed by others
References
Aarssen LW (1997) High productivity in grassland ecosystems: effected by species diversity or productive species? Oikos 80(1):183–184
Alvarez M, Pardo I (2005) Life history and production of Agapetus quadratus (Trichoptera: Glossosomatidae) in a temporary, spring-fed stream. Freshw Biol 50:930–943. doi:10.1111/j.1365-2427.2005.01370.x
Ballinger A, Lake PS (2006) Energy and nutrient fluxes from rivers and streams into terrestrial food webs. Mar Freshw Res 57:15–28
Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156
Batzer DP, Palik BJ (2007) Variable response by aquatic invertebrates to experimental manipulations of leaf litter input into seasonal woodland ponds. Fund Appl Limnol 168(2):155–162
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:201–220. doi:10.1111/j.1365-2427.2004.01328.x
Benke AC (1993) Concepts and patterns of invertebrate production in running waters. In: Congress of the International Assoc. of Theoretical and Applied Limnology, Barcelona, Spain, 1993. International association of theoretical and applied limnology proceedings. pp 15–38
Binckley CA, Resetarits WJ Jr (2007) Effects of forest canopy on habitat selection in treefrogs and aquatic insects: implications for communities and meta-communities. Oecologia 153:951–958
Bouchard V, Frey SD, Gilbert JM, Reed SE (2007) Effects of macrophyte functional group richness on emergent freshwater wetland functions. Ecology 88(11):2903–2914
Burton TM, Likens GE (1975) Salamander populations and biomass in Hubbard Brook experimental forest, New Hampshire. Copeia 3:541–546
Cadenasso ML, Weathers KC, Pickett STA (2004) Integrating food web and landscape ecology: subsidies at the regional scale. In: Polis GA, Power ME, Huxel GR (eds) Food webs at the landscape level. The University of Chicago Press, Chicago, pp 263–267
Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443(7114):989–992
Davic RD, Welsh HH (2004) On the ecological roles of salamanders. Ann Rev Ecol Evol Sys 35:405–434
Dreyer J, Hoekman D, Gratton C (2012) Lake-derived midges increase abundance of shoreline terrestrial arthropods via multiple trophic pathways. Oikos 121:252–258
Duffy JE (2002) Biodiversity and ecosystem function: the consumer connection. Oikos 99(2):201–219
Earl JE, Luhring TM, Williams BK, Semlitsch RD (2011) Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshw Biol 56:2473–2482
Edwards FK, Lauridsen RB, Armand L, Vincent HM, Jones JI (2009) The relationship between length, mass and preservation time for three species of freshwater leeches (Hirudinea). Fund Appl Limnol 173(4):321–327
Fisher SG, Likens GE (1973) Energy flow in Bear Brook, New Hampshire: integrative approach to stream ecosystem metabolism. Ecol Monogr 43(4):421–439
Gibbons JW, Bennett DH (1974) Determination of anuran terrestrial activity patterns by a drift fence method. Copeia 1:236–243
Gibbons JW, Winne CT, Scott DE, Willson JD, Glaudas X, Andrews KM, Todd BD, Fedewa LA, Wilkinson L, Tsaliagos RN, Harper SJ, Greene JL, Tuberville TD, Metts BS, Dorcast ME, Nestor JP, Young CA, Akre T, Reed RN, Buhlmann KA, Norman J, Croshaw DA, Hagen C, Rothermel BB (2006) Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Con Biol 20(5):1457–1465
Gratton C, Vander Zanden MJ (2009) Flux of aquatic insect productivity to land: comparison of lentic and lotic ecosystems. Ecology 90(10):2689–2699
Gratton C, Donaldson J, Vander Zanden MJ (2008) Ecosystem linkages between lakes and the surrounding terrestrial landscape in Northeast Iceland. Ecosystems 11:764–774
Halverson MA, Skelly DK, Kiesecker JM, Freidenburg LK (2003) Forest mediated light regime linked to amphibian distribution and performance. Oecologia 134:360–364
Hoekman D, Dreyer J, Jackson RD, Townsend PA, Gratton C (2011) Lake to land subsidies: experimental addition of aquatic insects increases terrestrial arthropod densities. Ecology 92:2063–2072
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setala H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75(1):3–35
Hutchens JJ, Wallace JB (2002) Ecosystem linkages between southern Appalachian headwater streams and their banks: leaf litter breakdown and invertebrate assemblages. Ecosystems 5(1):80–91
Jackson JK, Fisher SG (1986) Secondary production, emergence, and export of aquatic insects of a Sonoran desert stream. Ecology 67(3):629–638
Kato Y, Hori M, Okuda N, Tayasu I, Takemon Y (2003) Spatial heterogeneity of trophic pathways in the invertebrate community of a temperate bog. Freshw Biol 55:450–462
Kitchell JF, Schindler DE, Herwig BR, Post DM, Olson MH, Oldham M (1999) Nutrient cycling at the landscape scale: the role of diel foraging migrations by geese at the Bosque del Apache National wildlife refuge, New Mexico. Limnol Oceanogr 44(3):828–836
Leroux SJ, Loreau M (2008) Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecol Lett 11(11):1147–1156
Leuven RSEW, Brock TCM, van Druten HAM (1985) Effects of preservation on dry- and ash-free dry weight biomass of some common aquatic macro-invertebrates. Hydrobiologia 127:151–159
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Ecology–Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294(5543):804–808
Loreau M, Mouquet N, Holt RD (2003) Meta-ecosystems: a theoretical framework for a spatial ecosystem ecology. Ecol Lett 6(8):673–679
Massol F, Gravel D, Mouquet N, Cadotte MW, Fukami T, Leibold MA (2011) Linking community and ecosystem dynamics through spatial ecology. Ecol Lett 14:313–323
McCoy MW, Barfield M, Holt RD (2009) Predator shadows: complex life histories as generators of spatially patterned indirect interactions across ecosystems. Oikos 118(1):87–100. doi:10.1111/j.1600-0706.2008.16878.x
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98(1):166–170. doi:10.1073/pnas.98.1.166
Norberg J (2004) Biodiversity and ecosystem functioning: a complex adaptive systems approach. Limnol Oceanogr 49(4):1269–1277
Nowlin WH, Vanni MJ, Yang LH (2008) Comparing resource pulses in aquatic and terrestrial ecosystems. Ecology 89(3):647–659
Palik B, Kastendick D (2010) Response of seasonal pond plant communities to upland forest harvest in northern Minnesota forests, USA. For Ecol Man 260:628–637
Palik B, Batzer D, Buech R, Nichols D, Cease K, Egeland L, Streblow D (2001) Seasonal pond characteristics across a chronosequence of adjacent forest ages in northern Minnesota. Wetlands 21:532–542
Palik B, Batzer D, Kern C (2006) Upland forest linkages to seasonal wetlands: litter flux, processing, and food quality. Ecosystems 9(1):142–151. doi:10.1007/s10021-005-0010-0
Paton PWC, Crouch WB (2002) Using the phenology of pond-breeding amphibians to develop conservation strategies. Con Biol 16(1):194–204. doi:10.1046/j.1523-1739.2002.00260.x
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Ann Rev Ecol Sys 28:289–316
Post DM, Taylor JP, Kitchell JF, Olson MH, Schindler DE, Herwig BR (1998) The role of migratory waterfowl as nutrient vectors in a managed wetland. Con Biol 12(4):910–920. doi:10.1046/j.1523-1739.1998.97112.x
Pough FH (1980) Advantages of ectothermy for tetrapods. Am Nat 115(1):92–112
Pray CL, Nowlin WH, Vanni MJ (2009) Deposition and decomposition of periodical cicadas (Homoptera: Cicadidae: Magicicada) in woodland aquatic ecosystems. J N Am Benthol Soc 28(1):181–195. doi:10.1899/08-038.1
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Regester KJ, Lips KR, Whiles MR (2006) Energy flow and subsidies associated with the complex life cycle of ambystomatid salamanders in ponds and adjacent forest in southern Illinois. Oecologia 147(2):303–314. doi:10.1007/s00442-005-0266-2
Romanuk TN, Vogt RJ, Young A, Tuck C, Carscallen MW (2010) Maintenance of positive diversity–stability relations along a gradient of environmental stress. PLoS One 5(4):1–9
Rubbo MJ, Kiesecker JM (2004) Leaf litter composition and community structure: translating regional species changes into local dynamics. Ecology 85(9):2519–2525
Rubbo MJ, Cole JJ, Kiesecker JM (2006) Terrestrial subsidies of organic carbon support net ecosystem production in temporary forest ponds: evidence from an ecosystem experiment. Ecosystems 9(7):1170–1176. doi:10.1007/s10021-005-0009-6
Rubbo MJ, Belden LK, Kiesecker JM (2008) Differential responses of aquatic consumers to variations in leaf-litter inputs. Hydrobiologia 605:37–44
Runck C (2007) Macro-invertebrate production and food web energetics in an industrially contaminated stream. Ecol Appl 17(3):740–753. doi:10.1890/05-1026
Sabo JL, Post DM (2008) Quantifying periodic, stochastic, and catastrophic environmental variation. Ecol Monogr 78(1):19–40
Sabo JL, Power ME (2002) Numerical response of lizards to aquatic insects and short-term consequences for terrestrial prey. Ecology 83:3023–3036
Schiesari L (2006) Pond canopy cover: a resource gradient for anuran larvae. Freshw Biol 51:412–423
Schlapfer F, Schmid B (1999) Ecosystem effects of biodiversity: a classification of hypotheses and exploration of empirical results. Ecol Appl 9(3):893–912. doi:10.2307/2641337
Schreiber S, Rudolf VHW (2008) Crossing habitat boundaries: coupling dynamics of ecosystems through complex life cycles. Ecol Lett 11(6):576–587. doi:10.1111/j.1461-0248.2008.01171.x
Skelly DK, Freidenburg LK, Kiesecker JM (2002) Forest canopy and the performance of larval amphibians. Ecology 83(4):983–992
Stagliano DM, Benke AC, Anderson DH (1998) Emergence of aquatic insects from two habitats in a small wetland of the southeastern USA: temporal patterns of numbers and biomass. J N Am Benthol Soc 17(1):37–53
Tilman D, Lehman CL, Thomson KT (1997) Plant diversity and ecosystem productivity: theoretical considerations. Proc Natl Acad Sci USA 94(5):1857–1861
Van Buskirk J (2009) Natural variation in morphology of larval amphibians: phenotypic plasticity in nature? Ecol Monogr 79:681–705
Van Buskirk J (2011) Amphibian phenotypic variation along a gradient in canopy cover: species differences and plasticity. Oikos 120:906–914
Vander Zanden MJ, Gratton C (2011) Blowin’ in the wind: reciprocal airborne carbon fluxes between lakes and land. Can J Fish Aquat Sci 68:170–182. doi:10.1139/F10-157
von Schiller D, Solimini AG (2005) Differential effects of preservation on the estimation of biomass of two common mayfly species. Arch Hydrobiol 164(3):325–334
Welborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Ann Rev Ecol Sys 27:337–363
Wesner JS (2010) Seasonal variation in the trophic structure of a spatial prey subsidy linking aquatic and terrestrial food webs: adult aquatic insects. Oikos 119(1):170–178
Wesner JS (2012) Emerging aquatic insects as predators in terrestrial systems across a gradient of stream temperature in North and South America. Freshw Biol 57:2465–2474
Whiles MR, Goldowitz BS (2001) Hydrologic influences on insect emergence production from central Platte River wetlands. Ecol Appl 11(6):1829–1842
Whiting DP, Whiles MR, Stone ML (2011) Patterns of macro-invertebrate production, trophic structure, and energy flow along a tallgrass prairie stream continuum. Limnol Oceanogr 56(3):887–898. doi:10.4319/lo.2011.56.3.0887
Williams DD (2006) The biology of temporary waters. Oxford University Press, Oxford
Acknowledgments
Construction of the drift fences and setting pitfall buckets could not have been completed without the help of: Katherine Bannar-Martin, Devin Bloom, Kristen Brochu, Kirsten Comberford, Maria Modanu, Stephen Pynn, David Stitt, and Caroline Tucker. We also thank Mark Conboy, Klara Jaspers-Fayer, Tristan Willis and Monica Candelaria for helping with field work. The tremendous dedication of Siao Ryan Yang and Ruby Sambi in processing samples is greatly appreciated. We thank Karen Pope for generously donating the emergence traps and Nathan Lovejoy for microscope and laboratory use. This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant awarded to DDW.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schriever, T.A., Cadotte, M.W. & Williams, D.D. How hydroperiod and species richness affect the balance of resource flows across aquatic-terrestrial habitats. Aquat Sci 76, 131–143 (2014). https://doi.org/10.1007/s00027-013-0320-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-013-0320-9