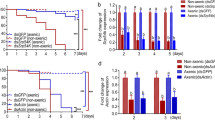
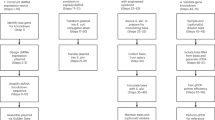
Abstract
RNA interference (RNAi) technology enables to study specific gene functions also in social insects, which are otherwise difficult to access for genetic manipulations. The recent sequencing of the genomes from seven ant species made these members of the Formicidae available for knockdown studies. However, for this purpose the RNAi technology first needs to be adapted for application in ants. Studies on other insects show that the effectiveness of RNAi is quite species-specific and can depend on several experimental parameters such as the investigated stage of the insect, the target gene and/or the dsRNA delivery method. RNAi in ants through feeding of dsRNA is a preferable approach, since knockdown can be achieved in individuals without interfering with the animal’s physiology in contrast to injection of dsRNA. Here, we present a protocol for gene knockdown in Formicidae by feeding of dsRNA to worker animals. The expression of a peptidoglycan recognition protein gene, PGRP-LB, was efficiently knocked down in the body of Camponotus floridanus worker ants. Moreover, we describe a relatively cheap method to extract dsRNA from bacteria in order to obtain large quantities needed for feeding experiments.



Similar content being viewed by others
References
Amdam G.V., Simoes Z.L., Guidugli K.R., Norberg K. and Omholt S.W. 2003. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnol. 3: 1
Bischoff V., Vignal C., Duvic B., Boneca I.G., Hoffmann J.A. and Royet J. 2006. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2: e14
Bonasio R., Zhang G., Ye C. et al. 2010. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 329: 1068-1071
Carlin N.F. and Hölldobler B. 1986. The kin recognition system of carpenter ants (Camponotus spp.).1. Hierarchical cues in small colonies. Behav. Ecol. Sociobiol. 19: 123-134
Choi M.Y. and Vander Meer R.K. 2012. Ant trail pheromone biosynthesis is triggered by a neuropeptide hormone. PLoS ONE 7: e50400
Choi M.Y., Vander Meer R.K., Coy M. and Scharf M.E. 2012. Phenotypic impacts of PBAN RNA interference in an ant, Solenopsis invicta, and a moth, Helicoverpa zea. J. Insect Physiol. 58: 1159-1165
Erthal M., Jr., Silva P.C. and Samuels I.R. 2007. Digestive enzymes in larvae of the leaf cutting ant, Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini). J. Insect Physiol. 53: 1101-1111
Feinberg E.H. and Hunter C.P. 2003. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 301: 1545-1547
Feldhaar H., Straka J., Krischke M., Berthold K., Stoll S., Mueller M.J. and Gross R. 2007. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 5: 48
Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E. and Mello C.C. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806-811
Hamilton C., Lejeune B.T. and Rosengaus R.B. 2011. Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus. Biol. Lett. 7: 89-92
Hölldobler B. and Wilson E.O. 1990. The Ants. Belknap Press of Harvard University Press, Cambridge, Mass
Huvenne H. and Smagghe G. 2010. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J. Insect Physiol. 56: 227-235
Ihle K.E., Page R.E., Frederick K., Fondrk M.K. and Amdam G.V. 2010. Genotype effect on regulation of behaviour by vitellogenin supports reproductive origin of honeybee foraging bias. Anim. Behav. 79: 1001-1006
Jarosch A. and Moritz R.F. 2011. Systemic RNA-interference in the honeybee Apis mellifera: tissue dependent uptake of fluorescent siRNA after intra-abdominal application observed by laser-scanning microscopy. J. Insect Physiol. 57: 851-857
Kamath R.S. and Ahringer J. 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313-321
Kamath R.S., Martinez-Campos M., Zipperlen P., Fraser A.G. and Ahringer J. 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002
Kulkarni M.M., Booker M., Silver S.J., Friedman A., Hong P., Perrimon N. and Mathey-Prevot B. 2006. Evidence of off-target effects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat. Methods 3: 833-838
Livak K.J. and Schmittgen T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408
Lu H.L., Vinson S.B. and Pietrantonio P.V. 2009. Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS J. 276: 3110-3123
Martinez J., Patkaniowska A., Urlaub H., Luhrmann R. and Tuschl T. 2002. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110: 563-574
Mello C.C. and Conte D., Jr. 2004. Revealing the world of RNA interference. Nature 431: 338-342
Mellroth P., Karlsson J. and Steiner H. 2003. A scavenger function for a Drosophila peptidoglycan recognition protein. J. Biol. Chem. 278: 7059-7064
Mellroth P. and Steiner H. 2006. PGRP-SB1: an N-acetylmuramoyl l-alanine amidase with antibacterial activity. Biochem. Biophys. Res. Commun. 350: 994-999
Mito T., Nakamura T., Bando T., Ohuchi H. and Noji S. 2011. The advent of RNA interference in Entomology. Entomol. Sci. 14: 1-8
Nelson C.M., Ihle K.E., Fondrk M.K., Page R.E. and Amdam G.V. 2007. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5: e62
Nunes F.M. and Simoes Z.L. 2009. A non-invasive method for silencing gene transcription in honeybees maintained under natural conditions. Insect Biochem. Mol. Biol. 39: 157-160
Nygaard S., Zhang G., Schiott M. et al. 2011. The genome of the leaf-cutting ant Acromyrmex echinatior suggests key adaptations to advanced social life and fungus farming. Genome Res. 21: 1339-1348
Paredes J.C., Welchman D.P., Poidevin M. and Lemaitre B. 2011. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity 35: 770-779
Patel A., Fondrk M.K., Kaftanoglu O., Emore C., Hunt G., Frederick K. and Amdam G.V. 2007. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE 2: e509
Rajagopal R., Sivakumar S., Agrawal N., Malhotra P. and Bhatnagar R.K. 2002. Silencing of midgut aminopeptidase N of Spodoptera litura by double-stranded RNA establishes its role as Bacillus thuringiensis toxin receptor. J. Biol. Chem. 277: 46849-46851
Ratzka C., Förster F., Liang C., Kupper M., Dandekar T., Feldhaar H. and Gross R. 2012. Molecular characterization of antimicrobial peptide genes of the carpenter ant Camponotus floridanus. PLoS ONE 7 (8): e43036
Ratzka C., Gross R. and Feldhaar H. 2013. Gene expression analysis of the endosymbiont-bearing midgut tissue during ontogeny of the carpenter ant Camponotus floridanus. J. Insect Physiol. 59: 611-623
Ratzka C., Liang C., Dandekar T., Gross R. and Feldhaar H. 2011. Immune response of the ant Camponotus floridanus against pathogens and its obligate mutualistic endosymbiont. Insect Biochem. Mol. Biol. 41: 529-536
Rodriguez-Cabrera L., Trujillo-Bacallao D., Borras-Hidalgo O., Wright D.J. and Ayra-Pardo C. 2010. RNAi-mediated knockdown of a Spodoptera frugiperda trypsin-like serine-protease gene reduces susceptibility to a Bacillus thuringiensis Cry1Ca1 protoxin. Environ. Microbiol. 12: 2894-2903
Rozen S. and Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132: 365-386
Sauer C., Dudaczek D., Hölldobler B. and Gross R. 2002. Tissue localization of the endosymbiotic bacterium “Candidatus Blochmannia floridanus” in adults and larvae of the carpenter ant Camponotus floridanus. Appl. Environ. Microbiol. 68: 4187-4193
Schlüns H. and Crozier R.H. 2007. Relish regulates expression of antimicrobial peptide genes in the honeybee, Apis mellifera, shown by RNA interference. Insect Mol. Biol. 16: 753-759
Smith C.D., Zimin A., Holt C. et al. 2011a. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl Acad. Sci. USA 108: 5673-5678
Smith C.R., Smith C.D., Robertson H.M. et al. 2011b. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc. Natl Acad. Sci. USA 108: 5667-5672
Stoll S., Feldhaar H. and Gross R. 2008. Transcriptional profiling of the endosymbiont Blochmannia floridanus during different developmental stages of its holometabolous ant host. Environ. Microbiol. 11: 877-888
Suen G., Teiling C., Li L. et al. 2011. The genome sequence of the leaf-cutter ant Atta cephalotes reveals insights into its obligate symbiotic lifestyle. PLoS Genet. 7: e1002007
Terenius O., Papanicolaou A., Garbutt J.S. et al. 2011. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 57: 231-245
Terra W.R., Espinozafuentes F.P. and Ferreira C. 1988. Midgut amylase, lysozyme, aminopeptidase, and trehalase from larvae and adults of Musca domestica. Arch. Insect Biochem. Physiol. 9: 283-297
Tian H., Peng H., Yao Q., Chen H., Xie Q., Tang B. and Zhang W. 2009. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 4: e6225
Timmons L., Court D.L. and Fire A. 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103-112
Timmons L. and Fire A. 1998. Specific interference by ingested dsRNA. Nature 395: 854
Walshe D.P., Lehane S.M., Lehane M.J. and Haines L.R. 2009. Prolonged gene knockdown in the tsetse fly Glossina by feeding double stranded RNA. Insect Mol. Biol. 18: 11-19
Wang Y., Mutti N.S., Ihle K.E., Siegel A., Dolezal A.G., Kaftanoglu O. and Amdam G.V. 2010. Down-regulation of honey bee IRS gene biases behavior toward food rich in protein. PLoS Genet. 6: e1000896
Winston W.M., Molodowitch C. and Hunter C.P. 2002. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456-2459
Wolschin F., Mutti N.S. and Amdam G.V. 2011. Insulin receptor substrate influences female caste development in honeybees. Biol. Lett. 7: 112-115
Wurm Y., Wang J., Riba-Grognuz O. et al. 2011. The genome of the fire ant Solenopsis invicta. Proc. Natl Acad. Sci. USA 108: 5679-5684
Zaidman-Remy A., Herve M., Poidevin M., Pili-Floury S., Kim M.S., Blanot D., Oh B.H., Ueda R., Mengin-Lecreulx D. and Lemaitre B. 2006. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 24: 463-473
Zaidman-Remy A., Poidevin M., Herve M., Welchman D.P., Paredes J.C., Fahlander C., Steiner H., Mengin-Lecreulx D. and Lemaitre B. 2011. Drosophila Immunity: Analysis of PGRP-SB1 Expression, Enzymatic Activity and Function. PLoS ONE 6: e17231
Zhu F., Xu J., Palli R., Ferguson J. and Palli S.R. 2011. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 67: 175-182
Acknowledgments
This work was funded by the priority program SFB567/C2 and the grant GR1243/8-1 of the Deutsche Forschungsgemeinschaft (DFG), and by the EU COST action FA0701 “Arthropod symbioses: from fundamental studies to pest management”. C. Ratzka was kindly supported by the program “Chancengleichheit für Frauen in Forschung und Lehre”. We thank Andreas Vilcinskas as well as Eileen Knorr for the opportunity to learn the application of RNAi at the University of Gießen. Moreover, Carolin Ratzka thanks Gro Amdam and Christina Grozinger for the chance to participate at the International Short Course on RNAi-Mediated Functional Genetics in Honey Bees.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ratzka, C., Gross, R. & Feldhaar, H. Systemic gene knockdown in Camponotus floridanus workers by feeding of dsRNA. Insect. Soc. 60, 475–484 (2013). https://doi.org/10.1007/s00040-013-0314-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-013-0314-6