Abstract
Extreme temperatures can constrain foraging behavior, and individual differences in thermal tolerances may affect foraging performance within and among species. Ambient temperatures may thus mediate competitive interactions among species that share resources. Different species of desert seed-harvesting ants (genus Messor) forage for similar food resources, and colonies can overlap in foraging areas. Because Messor species differ in body size distributions, and thermal tolerance is often size related in ants, we hypothesized that body size differences within and between Messor species would predict individual variation in worker thermal tolerances. Body size effects on thermal physiology could have implications for interspecific competition. We measured tolerances of extreme high (maximum critical temperature or CTmax) and low temperatures (CTmin) in two Messor species simultaneously at the same study site, smaller bodied M. ebeninus and larger bodied M. arenarius. Although the species did not differ significantly in CTmin or CTmax, tolerance of high temperatures was significantly size dependent for M. ebeninus: worker tolerances of high temperatures decreased with body size in this species. The patterns suggest the foraging activity of the smallest workers of smaller bodied species could be more constrained by high ambient temperatures, which could impact the division of labor within colonies as well as interspecific interactions.
Similar content being viewed by others
Introduction
Species differences in thermal physiology can affect interspecific ecological interactions. For example, when ambient temperatures approach or exceed a species’ thermal limits, that species may suffer decreased competitive ability relative to more thermally robust sympatric species. In some animal communities, species partition their activity periods depending on current thermal conditions (Cros et al. 1997; Kronfeld-Schor and Dayan 2003). The evolution of distinct species thermal physiologies is one mechanism that could favor species coexistence by reducing interspecific competition.
Desert seed-harvesting ants (genus Messor) are an excellent model system for testing how species differences in thermal physiology can affect resource use (Cerda and Retana 1994). Several Messor species co-occur in the northern Negev desert in Israel (Steinberger et al. 1991; Avgar et al. 2008). Messor species share food resources: the seeds of desert plants, with some overlap on harvesting from the same plant species (Steinberger et al. 1991). Although species’ nest sites can be statistically spatially segregated on a local scale (Warburg and Steinberger 1997), nests of different species often interdigitate, and the foraging areas used by colonies of different species often overlap (Avgar et al. 2008; Saar et al. 2018b). Therefore, these desert ants confront a high potential for interspecific competition for food among congeners. We hypothesize that species differences in thermal physiology could contribute to species coexistence among Negev desert Messor ants, and that species differences in thermal physiology could play an important role in regulating Messor species’ access to shared food resources.
Measuring thermal performance is an important first step in predicting how ambient temperatures can affect species differences in access to shared resources. Ants are small-bodied ectotherms, and ant workers do not socially thermoregulate when outside their nests. Across many environmental temperature ranges, ant workers’ bodies equilibrate with ambient temperature over the course of several seconds (Kaspari et al. 2015; O’Donnell unpub. data). Ant workers are, therefore, highly sensitive to ambient thermal conditions when they are foraging for food (Segev and Ziv 2012).
Species’ accessible thermal environments are bounded by low-temperature limits on activity (minimum critical temperature, or CTmin) and high-temperature limits (maximum critical temperature, or CTmax). Animals have an array of thermal performance curves that can differ within individuals: locomotion, digestion, etc. (Stevenson et al. 1985). CT measures set boundaries on organismal performance: at the extreme (high or low) temperatures where an organism loses the ability respond behaviorally, its diverse thermal performance curves effectively converge. CT measures provide a convenient bounding metric on subcaste thermal physiology differences, but low and high ambient temperatures that do not surpass CT values can negatively affect physiological performance in insects (Kaspari et al. 2015; Terblanche 2014; Sinclair et al. 2016). Therefore, CT differences can reflect individual variation in thermal physiology even at relatively moderate temperatures (Mesas et al. 2019).
Co-occurring Messor species differ in average worker body size and in the magnitude of size differences among nest mate workers (Segev et al. 2014). Although the relationship between body size and CTmax varies among ant taxa (Oberg et al. 2012), body size often correlates positively with CTmax both among and within ant species (Cerdá and Retana 1997; Ribeiro et al. 2012; Verble-Pearson et al. 2015; Wendt and Verble-Pearson 2016). Less is known about the relationship of CTmin with body size. This is an important consideration because CTmax and CTmin can vary independently within and among species (Hoffmann et al. 2013; Bishop et al. 2016): the patterns of body size effects on CTmax may not predict body size effects on CTmin within a species (Baudier and O’Donnell 2018). Body size differences in both CTmin and CTmax could affect patterns of task performance by workers, such as foraging activity times over the course of the day (Baudier and O'Donnell 2017).
To test whether sympatric Messor species differ in thermal physiology, we measured body size variation and critical thermal limits (CTmin and CTmax) in foraging workers of Messor arenarius and M. ebeninus in the northern Negev desert. We asked whether CTmin and CTmax differed between species and whether CT covaried with worker body size within species.
Methods
Subject collections
We collected worker ants and conducted thermal tolerance assays at the Jacob Blaustein Institute for Desert Research in Sde Boker, Israel from 31 July to 7 August 2019 (N 30° 52′ 24″, E 34° 47′ 35″). We surveyed the field station grounds to identify active nests of the seed-harvesting ants, Messor arenarius and M. ebeninus, recording latitude and longitude coordinates for each colony using hand-held GPS units (Fig. 1). A total of three nests of M. arenarius and five nests of M. ebeninus were used as sources of research subjects. Workers were collected from near the nest entrances with mouth aspirators. Ants were collected during early morning hours (0556 h to 0815 h local time), when most workers were returning to the nests after nighttime foraging trips. At collection, time and date were recorded, as well as ground temperature at the nest entrance using an infrared thermometer.
We collected sufficient workers each day (50–75 workers) to run simultaneous CTmax and CTmin trials on separate sets of workers from each colony. We selected workers spanning the apparent range of forager body sizes that were active at each colony. Thermal tolerance assays were initiated within 2 h of field collections to minimize the possibility of physiological acclimation by the worker ants (Oberg et al. 2012). Any collected workers that were not used in CT trials were returned to their nest entrances later the same day.
Thermal tolerance assays
On each day, we ran dynamic heat tolerance assays for CTmax and CTmin (Diamond et al. 2012; Ribeiro et al. 2012; Baudier and O’Donnell 2020) simultaneously on workers from one to two colonies. Assays were run on M. arenarius on August 1st, 2nd and 5th, and on M. ebeninus on August 4th, 6th, and 7th. During thermal assays, single workers were placed into capped 1.5-ml microcentrifuge tubes using soft forceps. A small piece of cotton was placed on the top of each tube to restrict the workers to the bottom 1.5 cm of the tube. Tubes were numbered and placed in aluminum blocks in the digital heating and cooling dry baths.
CTmax assays
We used a digital dry bath (Benchmark Scientific BSH1002) to manipulate temperatures. The aluminum blocks were pre-heated to a 30 °C starting temperature. After 5 min, each ant was checked for movement by lightly tapping the tube. We then increased the temperature of the heat block by 1 °C every 5 min and checked ants for movement after each 5-min interval at the new temperature. The highest temperature at which an ant responded to tapping with movement of any body part was recorded as its behavior and mobility-based CTmax (Diamond et al. 2012; Ribeiro et al. 2012; Baudier and O’Donnell 2020). We collected CTmax data on 89 M. arenarius workers from three colonies and from 90 M. ebeninus workers from five colonies.
CTmin assays
Workers for CTmin assays were nestmates of the CTmax workers, collected and assayed simultaneously. Tubes were placed into aluminum blocks in a Tropicooler (model 260014, Boekel Scientific) pre-cooled to 15 °C. After 5 min, ants in tubes were checked for movement, and the temperature was decreased by 1 °C. The lowest temperature at which an ant maintained movement (as above) was recorded as its CTmin. CTmin data were collected from 64 M. arenarius workers from three colonies, and from 65 M. ebeninus workers from five colonies.
Body size measurements
Head width was used as a measure of ant body size. Body mass and body length are strongly positively correlated with head width in both subject species (head width vs. body length log–log slope approximately 1.3 and linear regression R2 > 0.96 in both cases; Segev et al. 2014). Immediately following completion of thermal tolerance assays, each ant head was dissected from the body at the neck-like junction with the thorax (foramen). Heads were placed individually on a plain flat surface with the foramen facing down. Heads were photographed from directly above using a tripod-mounted digital camera at 4896 × 3672 pixel resolution (Sony Cybershot DSC-TX30). A ruler was included in each photograph to use as reference during head width measurement to convert pixels to millimeters. We measured head width in pixels at the widest point of the head excluding the eyes using the straight line tool in Image J version 153 (NIH 2020).
Statistical analyses
We used SPSS v. 26 software (IBM 2019) and Sigmaplot v. 12 software to analyze the data. We attempted to represent the body size (head width) range of workers we observed at nests in the workers we assayed in each colony. We tested whether the workers sampled for CT values differed in size among colonies within each species for both the CTmax and CTmin data sets. For this analysis, we used General Linear Models (GLM) to test for colony effects (as a random factor) on log10(head width), we also tested whether head width distributions differed between the species.
We calculated the linear regression relationships of CT (max and min) with head width for each species separately. If the linear relationship was significant, we tested whether adding a curvilinear (quadratic) term to the model significantly improved fit.
To identify significant predictors of CT values, we used GLM analyses. We log10 transformed head widths and CT values to improve linearity of data distributions; temperatures were converted to º K so they could be treated as scale variables. CT (max or min) values were the response variables, species identity was entered in the statistical model as a fixed factor, colony identity (nested within species) was entered as a random factor, and head width was entered as a covariate. We tested for the effects of species, colony, head width, and the (species x head width) interaction term (ANCOVA, testing whether species differed in the slope of the relationship of CT with head width) on CT (max or min).
Results
Worker body size differences within and between species
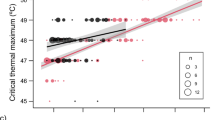
Within species, colonies did not differ significantly in head width distributions (M. ebeninus: CTmin samples F4,60 = 1.51, p = 0.21; CTmax samples F4,84 = 1.64, p = 0.17; M. arenarius: CTmin samples F2,61 = 0.80, p = 0.45; CTmax samples F2,86 = 0.48, p = 0.62). The two Messor species differed in worker body size: M. ebeninus workers were smaller on average than M. arenarius workers, although there was overlap in worker sizes (Fig. 2; for the CT max samples, species size difference F1,175 = 546.4, p < 0.001; for the CTmin samples, species size difference F1,127 = 281.4, p < 0.001).
Within-species body size effects on critical temperatures (CTmin and CTmax)
For the larger bodied species, M. arenarius, neither CTmin (r2 = 0.006, df = 62, p = 0.55) nor CTmax (R2 = 0.004, df = 86, p = 0.57) was significantly related to body size variation (Fig. 2).
For the smaller bodied species, M. ebeninus, CTmin decreased significantly with body size (R2 = 0.18, df = 63, p = 0.004), but adding a quadratic term did not improve model fit (R2 = 0.19; quadratic term t = − 0.47, p = 0.64). M. ebeninus CTmax increased significantly with body size (R2 = 0.19, df = 88, p < 0.0001), and the relationship was significantly curvilinear (Fig. 2; R2 = 0.26, df = 87, p < 0.0001; quadratic term t = − 2.77, p = 0.007).
Predictors of maximum critical temperature (CTmax)
The species did not differ significantly in overall CTmax distributions (F1,6 = 0.09, p = 0.78). CTmax increased with body size (F1,168 = 17.5, p < 0.001) but the species slopes for the relationship of CTmax with head width differed (interaction term F1,168 = 11.3, p < 0.005). One M. arenarius worker was identified as a significant CTmax outlier (stem-and-leaf analysis, p < 0.05) and excluded from the analyses; including this data point did not change any of the conclusions.
Predictors of minimum critical temperature (CTmin)
The species did not differ significantly in CTmin distributions (F1,6 = 0.007, p = 0.94). CTmin decreased with body size (F1,119 = 14.5, p < 0.001). The species had statistically similar slopes for the relationship of CTmin with head width (interaction term F1,119 = 1.9, p = 0.17).
Colony differences
Colonies differed significantly in CTmax values (F6,168 = 6.2, p < 0.001) and in CTmin values (F6,119 = 14.6, p < 0.001). Colony sample sizes were small for both species (M. arenarius, n = 3; M. ebeninus, n = 5), but there was no apparent relationship between colony mean CTmax and CTmin values for either species (Supp. Figure 1). Furthermore, the ground temperatures at the nest entrances when the ants were collected were moderate, not approaching worker CTmax or CTmin values, and ground temperature showed no apparent relationship with colony mean CT values (Supp. Figure 2).
Discussion
Species differences in size-related thermal physiology may be relevant to their ecological interactions. Although the two subject species did not differ significantly in overall mean CTmax, the species had different relationships of thermal tolerance with worker body size for CTmax. In the larger species, M. arenarius, worker body size did not predict CT variation. In the smaller species, M. ebeninus, smaller workers were less robust to extreme high temperatures. Within both species, colonies differed significantly in both CTmax and CTmin distributions. These colony differences were not caused by differences in body size distributions of the workers we sampled. Several factors could contribute to colony differences in thermal physiology, including colony age or developmental stage, short-term effects such as food intake (Bujan and Kaspari 2017), or genetic differences (Saar et al. 2018a).
We did not see evidence for temporal (or temperature-related) separation of species’ activity periods during our observations: all Messor species were foraging mainly at night during our data collection period, and foraging activity appeared to begin and end at similar times for the two subject species. Workers of both species, therefore, avoided the highest diurnal temperatures when foraging, minimizing the potential effects of species differences in CTmax on interspecific competitive interactions. However, both species forage during the day in other seasons when temperature effects on foraging may be stronger. When examined on an annual cycle, the two species exhibit substantial phenological overlap in their above-ground activity (Steinberger et al. 1992), but on a finer scale there is a clear temporal partitioning of activity across the diel cycle. In the spring and in the fall, M. ebeninus is mainly nocturnal whereas Messor arenarius forages mainly from dawn to early morning and from later afternoon until dusk (Avgar 2007; Giladi unpublished observations). Assuming the species differences in size–physiology relationships, we measured are not seasonally dependent (Bujan et al. 2020), this pattern suggests M. ebeninus may avoid foraging in conditions that could challenge the most vulnerable workers.
Messor arenarius workers were uniformly thermally robust across their entire body size range, but worker size variation could be important to thermal effects on behavior and division of labor in M. ebeninus. Social insect workers are often differentiated into specialized subcastes that perform distinct but complementary roles in colony function (Wills et al. 2018); in worker polymorphic species such as Messor ants, worker behavioral specialization is often associated with body size (Segev and Ziv 2012). Different thermal sensitivities among colony members, such as size subcastes, can cause conflicts over acceptable ambient temperature conditions. The most thermally vulnerable colony members may represent physiological weak links, and they may constrain some aspects of colony performance (Baudier and O’Donnell 2017). For example, smaller M. ebeninus workers may have a narrower tolerated range of ambient temperatures than their larger nestmates, as well as potential interspecific competitors. The relevance of within-colony size-related differences in thermal tolerance to M. ebeninus colony performance is not known, but worker size variation could be adaptive for other reasons that offset the thermal vulnerability of small workers. One possibility is that the more thermally vulnerable small workers bring competitive advantages, such as the ability to harvest seeds of different (presumably smaller) sizes or species, or the ability to collect seeds from different microhabitats.
An important caveat to interpreting our results is that the data were collected during a relatively hot and dry season in the Negev desert. Physiology data from other seasons are important to collect: some ant species workers show changes in thermal tolerances over time, such as across seasons (Bujan et al. 2020). However, the body size patterns we documented, particularly the greater thermal robustness of the larger species, accord well with findings on other ants. Several studies documented temperature-sensitive responses of the subject species well within the range bounded by the CT values. For example, Avgar (2007) found differences in running speed between M. arenarius and M. ebeninus with the former being faster overall (as might be expected based on body size) but also more temperature sensitive. The increase in running speed with ground temperature in M. arenarius was twice as fast as the increase in M. ebeninus. In another study, it was found that in both winter and summer, the range of temperatures at which M. arenarius visited seed baits was wider than that of M. ebeninus (Segev and Ziv 2012).
Two other aspects of these species’ foraging behavior may be related to their temperature tolerances and to the different size-tolerance patterns. The two species differ in their degree of sociality when foraging (Avgar et al. 2008). M. arenarius foragers mainly exhibit solitary foraging, whereas M. ebeninus forages in dense columns of workers. The efficiency of M. ebeninus foraging depends on the simultaneous activity of many workers which experience similar environmental conditions. Both species exhibit size-matching between forager size and food item size (Segev et al. 2014), a behavior which increases foraging efficiency when colonies encounter a diverse food supply (Traniello 1989; Retana and Cerda 1994). The extent to which these species can perform size-matching across a wide range of environmental conditions may be limited by body size–physiology relationships (Segev et al. 2014).
References
Avgar T (2007) Linking foraging traits of seed-eating ants to spatial patterns of surviving seeds. MS thesis, The Hebrew University of Jerusalem, Jerusalem
Avgar T, Giladi I, Nathan R (2008) Linking traits of foraging animals to spatial patterns of plants: social and solitary ants generate opposing patterns of surviving seeds. Ecol Lett 11:224–234
Baudier KM, O'Donnell S (2017) Weak links: how colonies counter the social costs of individual variation in thermal physiology. Current Opinion in Insect Science 22:85–91
Baudier KM, O'Donnell S (2018) Complex worker subcaste differences in thermal tolerances in the army ant Eciton burchellii parvispinum. J Therm Biol 77:278–280
Baudier KM, O’Donnell S (2020) Rain shadow effects predict population differences in thermal tolerance of leaf-cutting ant workers (Atta cephalotes). Biotropica 52:113–119
Bishop TR, Robertson MP, Rensburg BJ, Parr CL (2016) Coping with the cold: minimum temperatures and thermal tolerances dominate the ecology of mountain ants. Ecological Entomology 42:105–114
Bujan J, Kaspari M (2017) Nutrition modifies critical thermal maximum of a dominant canopy ant. J Insect Physiol 102:1–6
Bujan J, Roeder KA, Yanoviak SP, Kaspari M (2020) Seasonal plasticity of thermal tolerance in ants. Ecology 101:e03051
Cerda X, Retana J (1994) Food Exploitation Patterns of two sympatric seed-harvesting ants Messor bouvieri (Bond) and Messor capitatus (Latr) (Hym, Formicidae) from Spain. J Appl Entomol 117:268–277
Cerda X, Retana J (1997) Links between worker polymorphism and thermal biology in a thermophilic ant species. Oikos 78:467–474
Cros S, Cerda X, Retana J (1997) Spatial and temporal variations in the activity patterns of Mediterranean ant communities. Ecoscience 4:269–278
Diamond SE, Nichols LM, McCoy N, Hirsch C, Pelini SL, Sanders NJ, Ellison AM, Gotelli NJ, Dunn RR (2012) A physiological trait-based approach to predicting the responses of species to experimental climate warming. Ecology 93:2305–2312
Hoffmann AA, Chown SL, Clusella-Trullas S (2013) Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct Ecol 27:934–949
Kaspari M, Clay NA, Lucas J, Yanoviak SP, Kay A (2015) Thermal adaptation generates a diversity of thermal limits in a rainforest ant community. Glob Change Biol 21:1092–1102
Kronfeld-Schor N, Dayan T (2003) Partitioning of time as an ecological resource. Annu Rev Ecol Evol Syst 34:153–181
Mesas A, Jaramillo A, Castañeda LE (2019) Correlated evolution between heat tolerance and thermal performance curves in Drosophila subobscura. BioRxiv, 864793. https://doi.org/10.1101/864793
Oberg EW, Toro I, Pelini SL (2012) Characterization of the thermal tolerances of forest ants of New England. Insectes Soc 59:167–174
Retana J, Cerda X (1994) Worker size polymorphism conditioning size matching in two sympatric seed-harvesting ants. Oikos 71:261–266
Ribeiro PL, Camacho A, Navas CA (2012) Considerations for assessing maximum critical temperatures in small ectothermic animals: insights from leaf-cutting ants. PLoS ONE 7:e32083
Saar M, Eyer PA, Kilon-Kallner T, Hefetz A, Scharf I (2018a) Within-colony genetic diversity differentially affects foraging, nest maintenance, and aggression in two species of harvester ants. Scientific Reports 8:1–12
Saar M, Subach A, Reato I, Liber T, Pruitt JN, Scharf I (2018b) Consistent differences in foraging behavior in 2 sympatric harvester ant species may facilitate coexistence. Current Zoology 64:653–661
Segev U, Ziv Y (2012) Consequences of behavioral vs. numerical dominance on foraging activity of desert seed-eating ants. Behav Ecol Sociobiol 66:623–632
Segev U, Tielboerger K, Lubin Y, Kigel J (2014) Consequences of climate and body size on the foraging performance of seed-eating ants. Ecological Entomology 39:427–435
Sinclair BJ, Marshall KE, Sewell MA, Levesque DL, Willett CS, Slotsbo S, Dong Y, Harley CD, Marshall DJ, Helmuth BS, Huey RB (2016) Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol Lett 19:1372–1385
Steinberger Y, Leschner H, Shmida A (1991) Chaff piles of harvester ant (Messor spp.) nests in a desert ecosystem. Insectes Soc 38:241–250
Steinberger Y, Leschner H, Shmida A (1992) Activity pattern of harvester ants (Messor-Spp) in the Negev Desert ecosystem. J Arid Environ 23:169–176
Stevenson RD, Peterson CR, Tsuji JS (1985) The thermal dependence of locomotion, tongue flicking, digestion, and oxygen consumption in the wandering garter snake. Physiological Zoology 58:46–57
Terblanche JS (2014) Physiological performance of field-released insects. Current Opinion in Insect Science 4:60–66
Traniello JFA (1989) Foraging strategies of ants. Annu Rev Entomol 34:191–210
Verble-Pearson RM, Gifford ME, Yanoviak SP (2015) Variation in thermal tolerance of North American ants. J Therm Biol 48:65–68
Warburg I, Steinberger Y (1997) On the spatial distribution of nests of the ants Messor arenarius and Messor ebeninus. J Arid Environ 36:671–676
Wendt CFR, Verble-Pearson RM (2016) Critical thermal maxima and body size positively correlate in red imported fire ants, Solenopsis invicta. The Southwestern Naturalist 61:79–83
Wills BD, Powell S, Rivera MD, Suarez AV (2018) Correlates and consequences of worker polymorphism in ants. Annu Rev Entomol 63:575–598
Acknowledgements
Funding was provided by a Drexel University Stein Family Fellowship (to S. O’D.). This is publication no. 1086 of the Mitrani Department of Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Donnell, S., Bulova, S., Caponera, V. et al. Species differ in worker body size effects on critical thermal limits in seed-harvesting desert ants (Messor ebeninus and M. arenarius). Insect. Soc. 67, 473–479 (2020). https://doi.org/10.1007/s00040-020-00782-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-020-00782-5