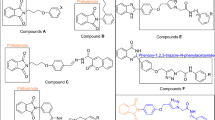
Abstract
In an effort to design and synthesize a new class of α-glucosidase and α-amylase inhibitors, we have synthesized novel pyrrole based molecules using molecular hybridization approach. These novel analogs were synthesized by the novel methodology developed in our lab which comprises of the multi-component direct synthesis route using hypervalent iodine reagent. The compounds were characterized by infrared, 1H nuclear magnetic resonance (NMR), 13C NMR and Mass Spectroscopy. These compounds were screened for their α-amylase and α- glucosidase activity. They showed a varying degree of inhibition with IC50 values ranging between 0.4 to 4.14 µmol/mL and 0.8 to 4.14 µmol/mL for α-amylase and α-glucosidase respectively. Compounds 3, 7, 12, and 18 showed excellent activity as compared to standard acarbose. This has identified a new class of α-amylase and α-glucosidase inhibitor which can be further developed as antihyperglycemic agents. The molecular docking analysis was carried out to better understand of interaction between α-amylase and α-glucosidase target and inhibitors in this series. We also generated a homology model for human α-glucosidase enzyme and identified the key residues at the binding site. The outcome of the study could be used for the rational design of potent and selective α-amylase and α-glucosidase inhibitors, respectively.
Graphical abstract









Similar content being viewed by others
References
Adams SH (2011) Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2:445–456
Ali H, Houghton PJ, Soumyanath A (2006) α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes, with particular reference to Phyllanthus amarus. J Ethnopharmacol l107:449–455
Antre RV, Mane PB, Oswal RJ (2012) Antidiabetic drugs: an overview. J Pharm Chem Sci 1:301–306
Bruni CB, Sica V, Auricchio F, Covelli I (1970) Further kinetic and structural characterization of the lysosomal a-d-glucoside glucohydrolase from cattle liver. Biochimica et Biophysica Acta 212:470–477
Ballard TE, Cavanagh J, Huigens RW, Melander C, Moeller PDR, Richards JJ (2009) Evalution of dihydrooroidin as an antifouling additive in marine paint. Int Biodeterior Biodegrad 63:529–532
Bao-Le Li, Hai-Chuan Hu, Zhang Mo, Xia Dub, Zhang Zhan-Hui (2014) Nano- CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. N J chem 38:2435–2442
Dwek RA, Butters TD, Platt FM, Zitzmann N (2002) Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov 1:65–75
Florence NT, Benoit MZ, Alexandra T, Desire DD, Jonas K, Pierre K, Théophile D (2014) Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol 151:784–790
Glide 6.3, Schrödinger, LLC, New York, NY (2014) User manual
Goel A, Agarwal N, Singh FV, Sharon A, Tiwari P, Dixit M, Pratap R, Rama V, Srivastava AK, Maulik PR (2004) Antihyperglycemic activity of 2-methyl-3,4,5-triaryl-1H-pyrrolesin SLM and STZ models. Bioorg Med Chem Lett 14:1089–1092
Horii S (1986) Synthesis and alpha-d-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J Med Chem 29:1038–1046
Jadhav NC, Jagadhane PB, Patile HV, Telvekar VN (2013) Three-component direct synthesis of substituted pyrroles from easily accessible chemical moieties using hypervalent iodine reagent. Tetrahedron Lett 54:3019–3021
Kaushik P (2015) Bioassay guided fractionation and α-amylase inhibitory activity of flavanoid isolated from Pinus roxburghii Sarg. Nat Prod Chem Res 3:179
Khan KM, Rahim F, Wadood A, Kosar N, Tahad M, Lalania S, Khan A, Fakhria MI, Junaid M, Rehmanb W, Khan M, Perveen S, Sajid M, Choudharya MI (2014) Synthesis and molecular docking studies of potent α-glucosidase inhibitors based on biscoumarin skeleton. Eur J Med Chem 81:245–252
Kimura A, Lee HS, Lee JH, Lee IS, Park KH, Chiba S, Kim D (2004) Two potent competitive inhibitors discriminating alpha-glucosidase family I from family II. Carbohydr Res 339:1035–1040
LigPrep 2.3, Schrödinger, LLC, New York, NY (2014) User manual
Pegklidou K, Koukoulitsa C, Nicolaou I, Demopoulos VJ (2010) Design and synthesis of novel series of pyrrole based chemotypes and their evaluation as selective aldose reductase inhibitors. A case of bioisosterism between a carboxylic acid moiety and that of a tetrazole. J Bio &. Med Chem 18:2107–2114
QikProp, version 4.0, Schrödinger, LLC, New York, NY (2014) User manual
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–99
Sensl M, Priccl F, Rossl MG (1989) D-lysine effectively decreases the non-enzymic glycation of proteins in vitro. Clin Chem 35:384–387
Shantharam CS, Vardhan R, Gowda C, Sridhara D, Suhas MB, Suyoga DM (2013) Inhibition of protein glycation by urea and thiourea derivatives of glycine/proline conjugated benzisoxazole analogue-synthesis and structure–activity studies. Eur J Med Chem 60:325–332
Singh Dhanachandrah, Kirubakaran Palani, Nagarajan Shanthi, Muthusamy Karthikeyan, Sakkiah Sugunadevi (2007) Homology modeling, molecular dynamics, e-pharmacophore mapping and docking study of Chikungunya virus nsP2 protease. A semi empirical free energy force field with charge-based desolvation. J Comput Chem 28:1145–1152
Sulochana KN, Punitham R, Ramakrishnan S (1998) Beneficial effect of lysine and amino acids on cataractogenesis in experimental diabetes through possible antiglycation of lens proteins. Exp Eye Res 67:597–601
Sulochana KN, Ramakrishnan S, Rajesh M, Coral K, Badrinath SS (2001) Diabetic retinopathy: molecular mechanisms, present regime of treatment and future perspectives. Curr Sci 80:133–142
Tundis R (2010) Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini-Rev Med Chem 10:315–331
Viegas JC, Danuello A, Dasilva BV, Barreiro EJ, Fraga CA (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14:1829–1852
Wild, S, Roglic G, Green A, Sicree R, King H (2004) Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Xiao F, Chen S, Deng J, Du Y, Guo F, Guo Y, Li K, Sheng H, Yu J, Zhu J (2014) Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 63:841–850
Acknowledgements
The authors, N.C. Jadhav, N.V. Desai and V.N. Telvekar thanks University Grant Commission-New Delhi, India for providing fellowship under Special Assistant Programme (UGC-SAP) and finanacial support under UGC Major research scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jadhav, N.C., Pahelkar, A.R., Desai, N.V. et al. Design, synthesis and molecular docking study of novel pyrrole-based α-amylase and α-glucosidase inhibitors. Med Chem Res 26, 2675–2691 (2017). https://doi.org/10.1007/s00044-017-1965-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1965-z