Abstract
Background
Ultrasound (US)-guided biopsy is widely used for the diagnostic confirmation of focal lesions. For sampling of prostate tissue, magnetic resonance imaging (MRI)/US fusion-guided biopsy has already been implemented in routine clinical practice and has shown a superior detection rate of significant prostate cancer in risk assessment compared with standard systematic biopsy. Newer three-dimensional software tools with volumetric mapping of the prostate and biopsy core channels provide a better overview of systematic biopsy and thus contribute to more accurate treatment planning. Automatic fusion is a time-saver and can reduce potential examiner errors through greater standardization of the fusion process itself.
Methodical innovations
In abdominal pathologies, US fusion biopsy can improve the rate of successful tissue sampling by using fused imaging to target lesions that are barely visible or difficult to delineate on B‑mode US scans. In addition, solid portions within larger tumors with enhancement on contrast-enhanced US can be targeted selectively, thereby avoiding sampling of necrotic areas and improving the quality of tissue cores for histopathological work-up.
Conclusion
Especially in complex situations, use of US fusion not only saves time but also improves sampling accuracy, which in turn reduces the rate of insufficient tissue specimens that necessitate repeat biopsy.
Zusammenfassung
Hintergrund
Die gezielte Biopsie per Ultraschall (US) stellt einen weit verbreiteten Ansatz für die histologische Sicherung von fokalen Läsionen dar. Im Bereich der Prostatabiopsie ist die über eine Fusion von Magnetresonanztomographie (MRT) und US gesteuerte Biopsie bereits fest in die klinische Routine implementiert und hat eine überlegene Detektionsrate in Bezug auf das signifikante Prostatakarzinom im Vergleich zum systematischen Standardverfahren ergeben. Neuere 3‑D-Softwaretools mit volumetrischer Erfassung der Prostata und der entsprechenden Biopsiestichkanäle geben einen verbesserten Überblick über eine gezielte und systematische Biopsie für eine entsprechende Therapieplanung. Die automatische Fusion liefert eine Zeitersparnis und vermindert durch die erhöhte Standardisierung des Fusionsprozesses auch die Fehleranfälligkeit durch den Untersucher.
Methodische Innovationen
Bei abdominalen Eingriffen kann die US-Fusionsbiopsie die Rate erfolgreicher Biopsien verbessern, indem Läsionen, die im B‑Bild-US kaum sichtbar bzw. nur erschwert abgrenzbar sind, basierend auf der fusionierten Bildgebung gezielt punktiert werden können. Darüber hinaus kann eine selektive Biopsie von soliden, kontrastmittelaufnehmenden Arealen größerer Tumoren erfolgen und dadurch die Probengewinnung aus nekrotischen Bereichen innerhalb einer Raumforderung vermieden werden, wodurch die Qualität der Stanzen zur histopathologischen Aufarbeitung steigt.
Schlussfolgerung
Vor allem bei komplexen Biopsien kann neben der verbesserten Genauigkeit eine Zeitersparnis erreicht werden sowie mögliche Reinterventionen bei insuffizienten Ergebnissen vorausgegangener Biopsien vermieden werden.
Similar content being viewed by others
Ultrasound (US) plays a crucial role in the diagnostic algorithm of focal lesions in parenchymal organs. Ultrasound is widely available and rapid to perform, making it an ideal first-line imaging modality for patients with abdominal pathology. Moreover, contrast-enhanced US (CEUS) improves diagnostic performance in the detection and characterization of suspicious focal lesions in the liver, kidney, and spleen.
Besides its role in diagnostic imaging, US is a widely used tool for guiding biopsy from focal lesions for histological work-up. Targeted biopsy sampling with US guidance can improve the rate of adequate biopsies while at the same time avoiding complications such as organ perforation. Careful selection of suitable patients based on lesion size and localization is crucial, and attention should be paid to the patient’s coagulation status to reduce the risk of bleeding.
In addition to diagnostic procedures for tissue sampling, US guidance can be used for therapeutic interventions such as transarterial chemoembolization (TACE) or ablation therapies [1]. Another major advantage of US-based interventions is their flexibility and wide availability for bedside interventions, which can implement CEUS with its high temporal resolution of microbubble distribution in a dynamic process with permanent view of needle guidance [2].
Process of ultrasound image fusion
B‑mode US image fusion offers the potential for real-time imaging and can be combined with cross-sectional imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI). Furthermore, US image fusion can be combined with CEUS loops from the same examination using different contrast phases in order to define the target of interventional procedures [3].
Basically, a magnetic field generator and a compatible sensor on the transducer are required in addition to the high-performance ultrasound device. As a result, the position and orientation of the transducer can be captured [3]. With this equipment, preexisting DICOM datasets can be directly imported into the US machine. To match the imported images with the live US image, a target lesion is marked with a region of interest (ROI), and the examiner needs to find a comparable slice position on US. The fusion process can be performed manually by marking significant landmarks or by using an automated algorithm provided by the unit’s inbuilt software. As a result, the cross-sectional images of the DICOM dataset move along with the US live image. The additional use of specific US techniques such as CEUS or shear wave elastography (SWE) is possible without difficulties [3]. During dynamic US biopsy, the magnetic field generator should not be moved to avoid inadequate sensor tracking of the probe. Note that, because of the magnetic field generator, image fusion should not be used for abdominal interventions in patients with a pacemaker.
MRI/US fusion-guided biopsy of the prostate
The German S3 guideline for prostate cancer (PCa) provides detailed recommendations concerning diagnostic procedures including biopsy with sampling of 10–12 tissue cores guided by transrectal ultrasound (TRUS; [4]). The 2021 update of the guideline includes new recommendations for patients who are candidates for focal therapy of low-risk PCa. In these patients, it is especially important to make absolutely sure that they have focally confined PCa. The guideline recommends MRI/US fusion biopsy and systematic sampling to establish the diagnosis in these cases [4].
For risk assessment of PCa, the combination of preinterventional multiparametric MRI (mpMRI) and MRI-targeted biopsy or MRI/US fusion-guided biopsy is known to be superior to the standard procedure with TRUS-guided systematic sampling of 10–12 biopsies [5]. These results highlight the diagnostic importance of MRI/US fusion biopsies. Several studies show that fusion biopsy detects more high-risk PCa, also in patients who undergo repeat biopsy or in biopsy-naïve patients [6,7,8]. Another study indicates that perfusion parameters on CEUS such as higher peak intensity or wash-in area can be used to differentiate histologically malignant and benign lesions and to assess tumor aggressiveness [9]. Thus, multiparametric US (mpUS) parameters provide additional information for risk assessment of pre-defined lesions sampled by target biopsy. Furthermore, SWE and tissue Doppler imaging (TDI) can help confirm suspected PCa in MRI-predefined areas during TRUS, further extending the use of the known submodalities in the context of mpUS [10, 11].
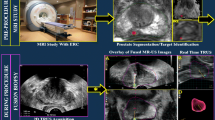
In their recent study, Das et al. conclude that fusion itself is the most decisive part of the whole examination process, emphasizing that an automated procedure can be chosen for fusion [12]. State-of-the-art software tools for volumetric assessment of the prostate—which are particularly advantageous when used with three-dimensional (3D) TRUS datasets—allow for fully automatic correlation of mpMRI datasets with a display of the ROI in dynamic TRUS. During the biopsy procedure, the needle tip position for each biopsy sample (both targeted and systematic biopsy) can be marked by an “X” (Fig. 1b). Afterwards, the initially defined volume is available for contouring of the prostate gland (Fig. 1c) including the ROI and the sites of tissue sampling in a 3D display. This makes it possible to visualize a 3D projection of all core channels and their spatial relationship to the ROI and, in the case of an additional systematic biopsy, to the prostate as a whole (Fig. 1d). Automatic fusion is a time-saver while simultaneously reducing any potential operator bias by greater standardization of the fusion process itself [12]. Volumetric assessment and fusion imaging are very helpful for interventional planning, for example, in a transperineal approach during irreversible electroporation of low-grade PCa [13]. In summary, the current role of fusion biopsy of the prostate is receiving considerable scientific attention, and the available results are mostly positive. We expect further advances and technical refinements in the near future, which, above all, are expected to lead to further automation and standardization of the examination procedure.
Magnetic resonance imaging/ultrasound fusion-guided prostate biopsy with corresponding three-dimensional visualization. a Ultrasound shows a hypoechogenic lesion in the left peripheral zone with hypervascularization on mircroflow imaging. b Targeted biopsy of the predefined region of interest (ROI); a green x is placed to indicate the needle tip position after the biopsy core has been obtained. c Edges of the prostate gland are outlined for volumetric assessment of the prostate gland in four different planes. d 3D volume of the prostate gland and the ROI used for target biopsy (pink ball) and visualization of all 12 core biopsies
Apart from the prostate, US fusion biopsy can also be used in transrectal biopsy of patients with perirectal lesions or recurrent tumor manifestations. The fusion process is comparable to the procedure for prostate fusion biopsy in the transverse plane with 3D imaging of the combined target area (e.g., the site of recurrent tumor) and biopsy channels allowing for documentation and confirmation of adequate tissue sampling (Fig. 2).
Magnetic resonance imaging (MRI)/ultrasound fusion-guided biopsy of recurrent low-grade ovarian cancer after surgery. a MRI shows recurrence after surgery of an ovarian borderline tumor. b Fusion-guided biopsy showing the coronal and axial planes of MRI with the region of interest (ROI) for targeted biopsy. c B-mode image and 3D volume (inset) showing biopsy channels within the target ROI, confirming successful sampling of the target lesion. Histopathology confirmed the diagnosis
Abdominal and retroperitoneal interventions
In the hands of experienced examiners, freehand biopsy is more successful in smaller lesions because it allows for a wider range of motion compared with fixed needle-guided interventions. Ahn et al. reported a statistically significant improvement in diagnostic success for fusion-guided biopsy (94.4% vs. 83%, p < 0.03). In addition, the authors noted a shorter intervention time (p < 0.02) and reported a significant number of lesions that became exclusively visible by fusion, while they could not be reliably delineated in conventional B‑mode US [14].
Ultrasound image fusion with CT/MRI offers several advantages in interventional procedures: It facilitates targeted sampling of lesions that are difficult to see in B‑mode US and improves selective biopsy of solid enhancing tissue portions in larger retroperitoneal tumors such as renal cell carcinoma or primary and metastatic liver lesions. It is also an important tool for documentation, since stored US images showing the biopsy sites can confirm adequate tissue sampling, especially in complex cases. In interventional procedures of renal cancer (especially in smaller lesions), fusion imaging may be helpful in identifying renal lesions hardly visible on B‑mode imaging and in planning the adequate procedure; kidney biopsies, on the other hand, are a generally feasible approach for US due to their retroperitoneal localization and an optimal acoustic window without interposition of air-containing structures [15]. It has been shown that, for focal liver tumors invisible in B‑mode US, the detection rate of suspicious focal liver lesions also increases with diagnostic CEUS compared with contrast-enhanced CT [16].
Apart from cross-sectional image fusion, immediate preinterventional CEUS with an additional live US scan to reliably identify the lesion during the intervention can also improve the rate of successful biopsies (Fig. 3). Both CT/MRI-CEUS and US-CEUS fusion imaging are feasible approaches for intraprocedural evaluation of treatment response in thermal ablation of focal liver lesions, while US-CEUS fusion imaging has shown a higher success rate in image registration [17].
Contrast-enhanced ultrasound (CEUS)/US-guided liver biopsy in a patient with suspected liver metastases. a Late contrast phase after intravenous Primovist (Bayer) injection shows a small (8 mm) liver lesion in the right hepatic lobe (segment IV). b The lesion is difficult to detect in the B‑mode US scan (right image) while it is clearly seen in the late contrast phase after administration of 1.6 ml Sonovue (Bracco Imaging). The lesion is marked by a region of interest (ROI, pink circle). The area was confirmed to contain solid enhancing tissue with enhancement during the arterial phase, which would not be present in necrotic tissue (no enhancement). c Dynamic fusion allows for dynamic targeted biopsy of the ROI transferred to B‑mode US from prior CEUS images. Histopathology confirmed metastasis from pancreatic cancer
Demarcation of focal lesions can be assessed during the portal venous or late contrast phase of a CEUS examination using a sensor-based 3D dataset and stored as a dataset for image fusion with dynamic B‑mode US (Fig. 3). As demonstrated by Rennert and colleagues, image fusion with volume navigation of CEUS and CT or MRI allows for the definite localization and diagnosis of hepatic lesions (both primary hepatic carcinoma and metastatic disease) based on continuous documentation of their contrast enhancement pattern [3]. The findings can lead to a change in therapeutic strategy in some patients with multiple lesions.
On dynamic US biopsy, a cross-sectional imaging DICOM dataset can be used to place an ROI around the target lesion and then fused to the live image during the B‑mode US scan. On dynamic US biopsy, additional options during dynamic US biopsy and the fusion process include color-coded Doppler US (CDUS) and microflow imaging (Fig. 4). This allows for continuous tracking of a lesion and confirmation of the target area identified by an ROI combined with a lower complication rate. The combination of CEUS-guided fusion biopsy and needle tracking offers the most sensitive targeting and sampling of a lesion within a predefined ROI (e.g., tumor lesion). Moreover, biopsy can be performed with additional microflow imaging even when a lesion is invisible in B‑mode US (Fig. 4c, d).
Contrast-enhanced ultrasound (CEUS)/US fusion-guided biopsy of a renal lesion in a patient with known Kaposi syndrome. a, b Unclear focal renal lesion not apparent in nonenhanced T2-weighted image (a) but with clear washout in contrast-enhanced T1-weighted image (b). c Invisible lesion on B‑mode US with washout in CEUS (inset) one minute after administration of contrast agent. d 3D sensor-based image loop of the whole kidney obtained during late phase 2 min after injection of contrast medium. e In the fused CEUS/US image, the lesion is marked by a region of interest (ROI) and confirmed by low vascularity on superb microvascular imaging. f Needle tracking facilitates targeted biopsy of the ROI. Biopsy confirmed adrenal gland tissue within the kidney, which was found to be stable at the 12-month follow-up
Regardless of whether CEUS/US, CT/US or MRI/US is used for fusion, depiction of the necrotic area (e.g., lack of enhancement) in contrast-enhanced imaging facilitates selective sampling of solid marginal tumor areas, thus improving the chance of obtaining a useful tissue sample for histopathological work-up (Fig. 5). In such cases, needle tracking supports the examiner and increases the accuracy of the procedure (Fig. 5b). Needle tracking can be performed using special needles provided by the company (with implemented sensor) or with generally available needles and additional, externally fixable sensor. During the fusion biopsy, the predefined ROI can be used as a target for advancement of the needle guided by US, even if part of the needle is not visible on in-plane imaging (Fig. 6). This technique shows the optimal needle track to sample the target lesion with a guidance line, while the actual needle position can be tracked by 3D sensor-based imaging. In 2013, Hakime and colleagues reported that targeted US liver biopsy performed with electromagnetic needle tracking had a slightly higher success rate than the standard procedure. In addition, needle tracking reduced the time required for correct needle alignment [18]. Thus, needle tracking can also contribute to more patient comfort, especially in complex cases, where successful biopsy may be difficult when only B‑mode US is used.
Contrast-enhanced ultrasound (CEUS)-guided biopsy in a patient with suspected large renal cell carcinoma. a Large renal tumor with central necrosis (area without contrast enhancement) marked by a freehand region of interest (ROI) on contrast-enhanced computed tomography. b Needle tracking is used to target the solid enhancing tissue area (green dot) identified by CEUS in the corresponding B‑mode image by guiding the tip of the needle (white arrowhead) to the target zone
Fusion biopsy of the liver with needle tracking. a Preinterventional marking of a liver tumor on magnetic resonance imaging (MRI) in three different planes for volumetric reconstruction. b Needle tracking shows the course of the needle (green line) on MRI and the target lesion (outlined in blue). The dotted yellow lines indicate the desired needle track, while the green dotted line indicates the actual needle position (right image, B‑mode ultrasound), which should always be within the track delimited by the yellow dotted lines
Overall, US fusion biopsy is well established in clinical practice for prostate biopsy, while 3D volumetry takes treatment planning to a new level. In abdominal interventions, fusion biopsy can improve successful tissue sampling in complex cases and targeting of lesions difficult to identify with B‑mode US alone.
Further indications for US fusion imaging
Similar to the procedure of abdominal and retroperitoneal biopsies, US fusion biopsy can be helpful in US-guided percutaneous drainage of complex fluid collections. The retroperitoneally located pancreas is often difficult to visualize as an entire organ due to an interposition of hollow organs. In such cases, US fusion imaging offers improved visualization of the “blind area” that might not be clearly shown on normal B‑mode US [19]. While the clinical indications for fusion imaging of the pancreas can be summarized as guidance for biopsy or drainage and percutaneous treatment of pancreatic cancer, there are few studies and further clinical research is needed to confirm its validity [15]. While US-guided biopsy is increasingly used in peripheral lung lesions, US fusion imaging can improve the detection rate and identification of the minimal size of target lesions compared with B‑Mode US alone [20].
General limitations
Beside the aforementioned benefits, some limitations need to be addressed. Overall, fusion-guided interventions require mid-range to high-end US systems with adequate software and hardware tools (e.g., needles with tracking system or biopsy introducer set). This equipment is more expensive than standardized core biopsy needles and the fusion process leads to a longer intervention time. Moreover, image fusion is generally made with cognitive fusion based on anatomical landmarks, which requires greater technical experience by the investigator. Regular breathing maneuvers may influence the optimal overlap of real-time US images and merged tomographic images (CT/MRI), potentially leading to a lack of consistency of the slice position. In CEUS mode, the lower mechanical index leads to a lower B‑mode image quality, making the needle visualization during the biopsy process more challenging.
While the biopsy technique depends on the examiner’s skills and the accessibility of the target, US image fusion may be helpful for US-guided biopsy of lesions difficult to visualize on B‑mode US, which is encouraged in the EFSUMB guidelines on Interventional Ultrasound with strong consensus (100%; [21]).
Practical conclusion
-
Ultrasound (US) fusion biopsy can generally be performed using computed tomography or magnetic resonance imaging (MRI) DICOM datasets or pre-interventional contrast-enhanced US (CEUS) for image fusion.
-
Image fusion can be a useful option for targeting organ lesions difficult to identify on B‑mode US.
-
In large tumors, CEUS/US image fusion can improve successful sampling of solid tissue while avoiding necrotic areas.
-
Detection of cancer can be improved with MRI/US image fusion and may contribute to better treatment planning when combined with state-of-the-art 3D visualization of the biopsy procedure.
References
Jung EM, Clevert DA (2018) Contrast-enhanced ultrasound (CEUS) and image fusion for procedures of liver interventions. Radiologe 58(6):538–544. https://doi.org/10.1007/s00117-018-0411-7
Rennert J, Georgieva M, Schreyer AG, Jung W, Ross C, Stroszczynski C et al (2011) Image fusion of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) using volume navigation for detection, characterization and planning of therapeutic interventions of liver tumors. Clin Hemorheol Microcirc 49(1-4):67–81. https://doi.org/10.3233/CH-2011-1458
Jung EM, Clevert DA (2015) Möglichkeiten der sonographischen Fusionsbildgebung: Aktuelle Entwicklungen. Radiologe 55(11):937–948. https://doi.org/10.1007/s00117-015-0025
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) (2021) S3-Leitlinie Prostatakarzinom, Langversion 6.0, AWMF Registernummer: 043/022OL. http://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom/. Accessed 11 April 2021
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH et al (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 378:1767–1777. https://doi.org/10.1056/NEJMoa1801993
Schlenker B, Apfelbeck M, Buchner A, Stief C, Clevert DA (2018) MRI-TRUS fusion biopsy of the prostate: quality of image fusion in a clinical setting. Clin Hemorheol Microcirc 70(4):433–440. https://doi.org/10.3233/CH-189308
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N et al (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 313(4):390–397. https://doi.org/10.1001/jama.2014.17942
Maxeiner A, Fischer T, Stephan C, Cash H, Slowinski T, Kilic E et al (2014) Die Echtzeit-MRT/US-Fusionsbiopsie verbessert die Detektionsrate des Prostatakarzinoms nach mehrfach negativen Vorbiopsien. Aktuelle Urol 45(3):197–203. https://doi.org/10.1055/s-0034-1375682
Maxeiner A, Fischer T, Schwabe J, Baur ADJ, Stephan C, Peters R et al (2019) Contrast-enhanced ultrasound (CEUS) and quantitative perfusion analysis in patients with suspicion for prostate cancer. Ultraschall Med 40(3):340–348. https://doi.org/10.1055/a-0594-2093
Maxeiner A, Fischer T, Stephan C, Treskatsch S, Baur ADJ, Jung E‑M et al (2021) Use of TDI during MRI/US fusion-guided biopsy for suspected prostate cancer. Clin Hemorheol Microcirc. https://doi.org/10.3233/CH-201035
Sidhu PS (2015) Multiparametric ultrasound (MPUS) imaging: terminology describing the many aspects of ultrasonography. Ultraschall Med 36:315–317. https://doi.org/10.1055/s-0035-1553381
Das CJ, Razik A, Netaji A, Verma S (2020) Prostate MRI–TRUS fusion biopsy: a review of the state of the art procedure. Abdom Radiol 45(7):2176–2183. https://doi.org/10.1007/s00261-019-02391-8
Baur ADJ, Collettini F, Enders J, Maxeiner A, Schreiter V, Stephan C et al (2017) MRI-TRUS fusion for electrode positioning during irreversible electroporation for treatment of prostate cancer. Diagn Interv Radiol 23(4):321–325. https://doi.org/10.5152/dir.2017.16276
Ahn SJ, Lee JM, Chang W, Lee SM, Kang HJ, Yang H‑K et al (2018) Clinical utility of real-time ultrasound-multimodality fusion guidance for percutaneous biopsy of focal liver lesions. Eur J Radiol 103:76–83. https://doi.org/10.1016/j.ejrad.2018.04.002
D’Onofrio M, Beleù A, Gaitini D, Corréas JM, Brady A, Clevert D (2019) Abdominal applications of ultrasound fusion imaging technique: liver, kidney, and pancreas. Insights Imaging 10:1–6. https://doi.org/10.1186/s13244-019-0692-z
Zhou Y, Wang Y, Wang F, Zhang X, Ding J, Zhou H et al (2021) Additional diagnostic value of fusion imaging of CEUS and first CEUS of invisible hepatic lesions ≤2 cm. J Ultrasound Med 40(6):1173–1181. https://doi.org/10.1002/jum.15498
Xu E, Long Y, Li K, Zeng Q, Tan L, Luo L et al (2019) Comparison of CT/MRI-CEUS and US-CEUS fusion imaging techniques in the assessment of the thermal ablation of liver tumors. Int J Hyperthermia 35(1):159–167. https://doi.org/10.1080/02656736.2018.1487591
Hakime A, Barah A, Deschamps F, Farouil G, Joskin J, Tselikas L et al (2013) Prospective comparison of freehand and electromagnetic needle tracking for us-guided percutaneous liver biopsy. J Vasc Interv Radiol 24(11):1682–1689. https://doi.org/10.1016/j.jvir.2013.05.044
Sumi H, Itoh A, Kawashima H, Ohno E, Itoh Y, Nakamura Y et al (2014) Preliminary study on evaluation of the pancreatic tail observable limit of transabdominal ultrasonography using a position sensor and CT-fusion image. Eur J Radiol 83(8):1324–1331. https://doi.org/10.1016/j.ejrad.2014.05.009
Imamine R, Kobayashi H, Akuta K, Matsuki M, Isoda H, Togashi K (2019) Diagnostic accuracy and complication rates of fusion images created using real-time ultrasound with CT for identification of peripheral lung lesions in patients undergoing biopsy. Open J Radiol 09:36–47. https://doi.org/10.4236/ojrad.2019.91004
Lorentzen T, Nolsoe CP, Ewertsen C, Nielsen MB, Leen E, Havre RF et al (2015) EFSUMB guidelines on interventional ultrasound (INVUS), part I: general aspects (short version). Ultraschall Med 36:464–472. https://doi.org/10.1055/s-0035-1553593
Acknowledgements
The authors thank Ms. Bettina Herwig for language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors reports a relationship with industry and other relevant entities—financial or otherwise—that might pose a conflict of interest in connection with the submitted article. The following authors report financial activities outside the submitted work: M.H. Lerchbaumer reports having received consultancy honoraria from Canon Medical Imaging and Siemens Healthineers. T. Fischer reports having received consultancy honoraria from Bracco, Siemens Healthineers, and Canon Medical Imaging.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
The supplement containing this article is not sponsored by industry.
Additional information

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Lerchbaumer, M.H., Fischer, T. Ultrasound fusion biopsy. Radiologe 61 (Suppl 1), 11–18 (2021). https://doi.org/10.1007/s00117-021-00893-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-021-00893-5