Abstract
Aims/hypothesis
Pantothenate kinase (PANK) is the first enzyme in CoA biosynthesis. Pank1-deficient mice have 40% lower liver CoA and fasting hypoglycaemia, which results from reduced gluconeogenesis. Single-nucleotide polymorphisms in the human PANK1 gene are associated with insulin levels, suggesting a link between CoA and insulin homeostasis. We determined whether Pank1 deficiency (1) modified insulin levels, (2) ameliorated hyperglycaemia and hyperinsulinaemia, and (3) improved acute glucose and insulin tolerance of leptin (Lep)-deficient mice.
Methods
Serum insulin and responses to glucose and insulin tolerance tests were determined in Pank1-deficient mice. Levels of CoA and regulating enzymes were measured in liver and skeletal muscle of Lep-deficient mice. Double Pank1/Lep-deficient mice were analysed for the diabetes-related phenotype and global metabolism.
Results
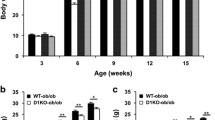
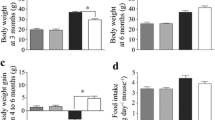
Pank1-deficient mice had lower serum insulin and improved glucose tolerance and insulin sensitivity compared with wild-type mice. Hepatic and muscle CoA was abnormally high in Lep-deficient mice. Pank1 deletion reduced hepatic CoA but not muscle CoA, reduced serum glucose and insulin, but did not normalise body weight or improve acute glucose tolerance or protein kinase B phosphorylation in Lep-deficient animals. Pank1/Lep double-deficient mice exhibited reduced whole-body metabolism of fatty acids and amino acids and had a greater reliance on carbohydrate use for energy production.
Conclusions/interpretation
The results indicate that Pank1 deficiency drives a whole-body metabolic adaptation that improves aspects of the diabetic phenotype and uncouples hyperglycaemia and hyperinsulinaemia from obesity in leptin-deficient mice.







Similar content being viewed by others
Abbreviations
- AKT:
-
Protein kinase B
- ITT:
-
Insulin tolerance test
- NUDT7:
-
Nudix hydrolase 7
- PANK:
-
Pantothenate kinase
- RER:
-
Respiratory exchange ratio
- \( \dot{V}{\mathrm{CO}}_2 \) :
-
Rate of CO2 production
- \( \dot{V}{\mathrm{O}}_2 \) :
-
Rate of O2 consumption
References
Perriello G, Pampanelli S, del Sindaco P et al (1997) Evidence of increased systemic glucose production and gluconeogenesis in an early stage of NIDDM. Diabetes 46:1010–1016
Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R (2010) Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol 205:97–106
Randle PJ (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14:263–283
Leonardi R, Rehg JE, Rock CO, Jackowski S (2010) Pantothenate kinase 1 is required to support the metabolic transition from the fed to the fasted state. PLoS ONE 5:e11107
Leonardi R, Zhang Y-M, Rock CO, Jackowski S (2005) Coenzyme A: back in action. Prog Lipid Res 44:125–153
Gasmi L, McLennan AG (2001) The mouse Nudt7 gene encodes a peroxisomal nudix hydrolase specific for coenzyme A and its derivatives. Biochem J 357:33–38
Reilly SJ, Tillander V, Ofman R, Alexson SE, Hunt MC (2008) The nudix hydrolase 7 is an acyl-CoA diphosphatase involved in regulating peroxisomal coenzyme A homeostasis. J Biochem 144:655–663
Zhang Y-M, Rock CO, Jackowski S (2005) Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters. J Biol Chem 280:32594–32601
Garcia M, Leonardi R, Zhang YM, Rehg JE, Jackowski S (2012) Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for CoA in postnatal metabolism. PLoS ONE 7:e40871
Rock CO, Karim MA, Zhang Y-M, Jackowski S (2002) The murine Pank1 gene encodes two differentially regulated pantothenate kinase isozymes. Gene 291:35–43
Leonardi R, Rock CO, Jackowski S, Zhang Y-M (2007) Activation of human mitochondrial pantothenate kinase 2 by palmitoylcarnitine. Proc Natl Acad Sci U S A 104:1494–1499
Leonardi R, Zhang YM, Lykidis A, Rock CO, Jackowski S (2007) Localization and regulation of mouse pantothenate kinase 2. FEBS Lett 581:4639–4644
Rock CO, Calder RB, Karim MA, Jackowski S (2000) Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J Biol Chem 275:1377–1383
Leonardi R, Zhang Y-M, Yun M-K et al (2010) Modulation of pantothenate kinase 3 activity by small molecules that interact with the substrate/allosteric regulatory domain. Chem Biol 17:892–902
Alfonso-Pecchio A, Garcia M, Leonardi R, Jackowski S (2012) Compartmentalization of mammalian pantothenate kinases. PLoS ONE 7:e49509
Gerdes S, Lerma-Ortiz C, Frelin O et al (2012) Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J Exp Bot 63:5379–5395
Zallot R, Agrimi G, Lerma-Ortiz C et al (2013) Identification of mitochondrial coenzyme a transporters from maize and Arabidopsis. Plant Physiol 162:581–588
Prohl C, Pelzer W, Diekert K et al (2001) The yeast mitochondrial carrier Leu5p and its human homologue Graves' disease protein are required for accumulation of coenzyme A in the matrix. Mol Cell Biol 21:1089–1097
Fiermonte G, Paradies E, Todisco S, Marobbio CM, Palmieri F (2009) A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′-diphosphate in human mitochondria. J Biol Chem 284:18152–18159
Zhang YM, Chohnan S, Virga KG et al (2007) Chemical knockout of pantothenate kinase reveals the metabolic and genetic program responsible for hepatic coenzyme A homeostasis. Chem Biol 14:291–302
Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ (2001) A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet 28:345–349
Sabatti C, Service SK, Hartikainen AL et al (2009) Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 41:35–46
Kennedy AJ, Ellacott KL, King VL, Hasty AH (2010) Mouse models of the metabolic syndrome. Dis Model Mech 3:156–166
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature (London) 372:425–432
Lindstrom P (2007) The physiology of obese-hyperglycemic mice [ob/ob mice]. Sci World J 7:666–685
Steiner AA, Petenusci SO, Brentegani LG, Branco LG (2000) The importance of glucose for the freezing tolerance/intolerance of the anuran amphibians Rana catesbeiana and Bufo paracnemis. Rev Bras Biol 60:321–328
Minkler PE, Kerner J, Ingalls ST, Hoppel CL (2008) Novel isolation procedure for short-, medium-, and long-chain acyl-coenzyme A esters from tissue. Anal Biochem 376:275–276
Shimada K, Mitamura K (1994) Derivatization of thiol-containing compounds. J Chromatogr B Biomed Appl 659:227–241
Schneider WC (1945) Phosphorus compounds in animal tissues; extraction and estimation of desoxypentose nucleic acid and of pentose nucleic acid. J Biol Chem 161:293–303
Yu XX, Drackley JK, Odle J, Lin X (1997) Response of hepatic mitochondrial and peroxisomal β-oxidation to increasing palmitate concentrations in piglets. Biol Neonate 72:284–292
Demaugre F, Leroux JP, Cartier P (1978) The effects of pyruvate concentration, dichloroacetate and alpha-cyano-4-hydroxycinnamate on gluconeogenesis, ketogenesis and [3-hydroxybutyrate]/[3-oxobutyrate] ratios in isolated rat hepatocytes. Biochem J 172:91–96
KirscHbAum N, Clemons R, Marino KA, Sheedy G, Nguyen ML, Smith CM (1990) Pantothenate kinase activity in livers of genetically diabetic mice (db/db) and hormonally treated cultured rat hepatocytes. J Nutr 120:1376–1386
Mutel E, Gautier-Stein A, Abdul-Wahed A et al (2011) Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes 60:3121–3131
Houten SM, Herrema H, Te BH et al (2013) Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Hum Mol Genet 22:5249–5261
Haymond MW, Miles JM (1982) Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 31:86–89
Newgard CB, An J, Bain JR et al (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326
Tai ES, Tan ML, Stevens RD et al (2010) Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53:757–767
Storlien L, Oakes ND, Kelley DE (2004) Metabolic flexibility. Proc Nutr Soc 63:363–368
Acknowledgements
We thank C. Pate, L. Richmond, K. Miller, J. Wang, K. Wells and M. Frank (Department of Infectious Diseases, St Jude Children’s Hospital), the Veterinary Pathology Core Facility at St Jude Children’s Hospital and the Protein Core of the University of California at Davis for their expert technical assistance.
Funding
The research was funded by NIH GM062896 (to SJ) and ALSAC.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
RL, SJ and COR were responsible for conception and design of the study. RL performed and supervised the experiments to obtain the data. RL and SJ were responsible for analysis and interpretation of the data, and wrote the manuscript. COR contributed to discussion and reviewed/edited the manuscript. All authors gave final approval. SJ is responsible for the integrity of the work as a whole.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 56 kb)
ESM Fig. 2
(PDF 45 kb)
Rights and permissions
About this article
Cite this article
Leonardi, R., Rock, C.O. & Jackowski, S. Pank1 deletion in leptin-deficient mice reduces hyperglycaemia and hyperinsulinaemia and modifies global metabolism without affecting insulin resistance. Diabetologia 57, 1466–1475 (2014). https://doi.org/10.1007/s00125-014-3245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3245-5