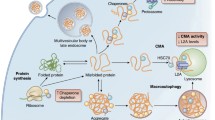
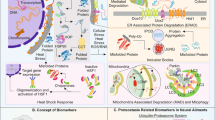
Abstract
In living organisms, proteins are regularly exposed to ‘molecular ageing’, which corresponds to a set of non-enzymatic modifications that progressively cause irreversible damage to proteins. This phenomenon is greatly amplified under pathological conditions, such as diabetes mellitus. For their survival and optimal functioning, cells have to maintain protein homeostasis, also called ‘proteostasis’. This process acts to maintain a high proportion of functional and undamaged proteins. Different mechanisms are involved in proteostasis, among them degradation systems (the main intracellular proteolytic systems being proteasome and lysosomes), folding systems (including molecular chaperones), and enzymatic mechanisms of protein repair. There is growing evidence that the disruption of proteostasis may constitute a determining event in pathophysiology. The aim of this review is to demonstrate how such a dysregulation may be involved in the pathogenesis of diabetes mellitus and in the onset of its long-term complications.



Similar content being viewed by others
Abbreviations
- FN3K:
-
Fructosamine-3-kinase
- HSP:
-
Heat shock protein
- MSR:
-
Methionine sulfoxide reductase
- PIMT:
-
Protein-l-isoaspartyl methyltransferase
- RAGE:
-
Receptor for AGE
- sHSP:
-
Small heat shock protein
- SNP:
-
Single-nucleotide polymorphism
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
References
Powers ET, Balch WE (2013) Diversity in the origins of proteostasis networks—a driver for protein function in evolution. Nat Rev Mol Cell Biol 14:237–248
Roth DM, Balch WE (2011) Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol 23:126–134
Gillery P, Jaisson S (2013) Usefulness of non-enzymatic post-translational modification derived products (PTMDPs) as biomarkers of chronic diseases. J Proteomics 92:228–238
Acharya JD, Ghaskadbi SS (2010) Islets and their antioxidant defense. Islets 2:225–235
Mohammedi K, Maimaitiming S, Emery N et al (2011) Allelic variations in superoxide dismutase-1 (SOD1) gene are associated with increased risk of diabetic nephropathy in type 1 diabetic subjects. Mol Genet Metab 104:654–660
Newsholme P, Rebelato E, Abdulkader F, Krause M, Carpinelli A, Curi R (2012) Reactive oxygen and nitrogen species generation, antioxidant defenses, and beta-cell function: a critical role for amino acids. J Endocrinol 214:11–20
Rabbani N, Thornalley PJ (2011) Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol 22:309–317
Hipkiss AR (2006) Accumulation of altered proteins and ageing: causes and effects. Exp Gerontol 41:464–473
Rock KL, Gramm C, Rothstein L et al (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761–771
Jung T, Catalgol B, Grune T (2009) The proteasomal system. Mol Aspects Med 30:191–296
Grimm S, Hohn A, Grune T (2012) Oxidative protein damage and the proteasome. Amino Acids 42:23–38
Chondrogianni N, Petropoulos I, Grimm S et al (2014) Protein damage, repair and proteolysis. Mol Aspects Med 35:1–71
Carrard G, Dieu M, Raes M, Toussaint O, Friguet B (2003) Impact of ageing on proteasome structure and function in human lymphocytes. Int J Biochem Cell Biol 35:728–739
Queisser MA, Yao D, Geisler S et al (2010) Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 59:670–678
Moheimani F, Morgan PE, van Reyk DM, Davies MJ (2010) Deleterious effects of reactive aldehydes and glycated proteins on macrophage proteasomal function: possible links between diabetes and atherosclerosis. Biochim Biophys Acta 1802:561–571
Grimm S, Ott C, Horlacher M, Weber D, Hohn A, Grune T (2012) Advanced-glycation-end-product-induced formation of immunoproteasomes: involvement of RAGE and Jak2/STAT1. Biochem J 448:127–139
Hartley T, Brumell J, Volchuk A (2009) Emerging roles for the ubiquitin-proteasome system and autophagy in pancreatic beta-cells. Am J Physiol Endocrinol Metab 296:E1–E10
Bugliani M, Liechti R, Cheon H et al (2013) Microarray analysis of isolated human islet transcriptome in type 2 diabetes and the role of the ubiquitin–proteasome system in pancreatic beta cell dysfunction. Mol Cell Endocrinol 367:1–10
Costes S, Huang CJ, Gurlo T et al (2011) Beta-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes 60:227–238
Wing SS (2008) The UPS in diabetes and obesity. BMC Biochem 9(Suppl 1):S6
Otoda T, Takamura T, Misu H et al (2013) Proteasome dysfunction mediates obesity-induced endoplasmic reticulum stress and insulin resistance in the liver. Diabetes 62:811–824
Liu H, Yu S, Xu W, Xu J (2012) Enhancement of 26S proteasome functionality connects oxidative stress and vascular endothelial inflammatory response in diabetes mellitus. Arterioscler Thromb Vasc Biol 32:2131–2140
Xu J, Wang S, Zhang M, Wang Q, Asfa S, Zou MH (2012) Tyrosine nitration of PA700 links proteasome activation to endothelial dysfunction in mouse models with cardiovascular risk factors. PLoS One 7:e29649
Aghdam SY, Gurel Z, Ghaffarieh A, Sorenson CM, Sheibani N (2013) High glucose and diabetes modulate cellular proteasome function: implications in the pathogenesis of diabetes complications. Biochem Biophys Res Commun 432:339–344
Gragnoli C (2011) Proteasome modulator 9 and macrovascular pathology of T2D. Cardiovasc Diabetol 10:1–4
Hermann J, Gulati R, Napoli C et al (2003) Oxidative stress-related increase in ubiquitination in early coronary atherogenesis. FASEB J 17:1730–1732
Marfella R, D’Amico M, Di Filippo C et al (2007) The possible role of the ubiquitin proteasome system in the development of atherosclerosis in diabetes. Cardiovasc Diabetol 6:35
Marfella R, Cacciapuoti F, Grassia A et al (2006) Role of the ubiquitin-proteasome system in carotid plaque instability in diabetic patients. Acta Cardiol 61:630–636
Hu J, Klein JD, Du J, Wang XH (2008) Cardiac muscle protein catabolism in diabetes mellitus: activation of the ubiquitin-proteasome system by insulin deficiency. Endocrinology 149:5384–5390
Doeppner TR, Mlynarczuk-Bialy I, Kuckelkorn U et al (2012) The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 135:3282–3297
Yu X, Patterson E, Kem DC (2009) Targeting proteasomes for cardioprotection. Curr Opin Pharmacol 9:167–172
Marfella R, D’Amico M, Esposito K et al (2006) The ubiquitin-proteasome system and inflammatory activity in diabetic atherosclerotic plaques: effects of rosiglitazone treatment. Diabetes 55:622–632
Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH (2007) Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 116:944–953
Friguet B, Szweda LI (1997) Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett 405:21–25
Rajawat YS, Hilioti Z, Bossis I (2009) Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev 8:199–213
Mehrpour M, Esclatine A, Beau I, Codogno P (2010) Overview of macroautophagy regulation in mammalian cells. Cell Res 20:748–762
Yamamoto A, Simonsen A (2011) The elimination of accumulated and aggregated proteins: a role for aggrephagy in neurodegeneration. Neurobiol Dis 43:17–28
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14
Dice JF (2007) Chaperone-mediated autophagy. Autophagy 3:295–299
Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH (2007) Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes 56:930–939
Xie Y, You SJ, Zhang YL et al (2011) Protective role of autophagy in AGE-induced early injury of human vascular endothelial cells. Mol Med Rep 4:459–464
Chen ZF, Li YB, Han JY et al (2011) The double-edged effect of autophagy in pancreatic beta cells and diabetes. Autophagy 7:12–16
Zhang X, Yuan Q, Tang W, Gu J, Osei K, Wang J (2011) Substrate-favored lysosomal and proteasomal pathways participate in the normal balance control of insulin precursor maturation and disposal in beta-cells. PLoS One 6:e27647
Hohn A, Konig J, Grune T (2013) Protein oxidation in aging and the removal of oxidized proteins. J Proteomics 92:132–159
Moheimani F, Kim CH, Rahmanto AS, van Reyk DM, Davies MJ (2012) Inhibition of lysosomal function in macrophages incubated with elevated glucose concentrations: a potential contributory factor in diabetes-associated atherosclerosis. Atherosclerosis 223:144–151
Jung HS, Chung KW, Won Kim J et al (2008) Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 8:318–324
Masini M, Bugliani M, Lupi R et al (2009) Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 52:1083–1086
Song YM, Song SO, You YH et al (2013) Glycated albumin causes pancreatic beta-cells dysfunction through autophagy dysfunction. Endocrinology 154:2626–2639
Patschan S, Goligorsky MS (2008) Autophagy: the missing link between non-enzymatically glycated proteins inducing apoptosis and premature senescence of endothelial cells? Autophagy 4:521–523
Liu H, Yu S, Zhang H, Xu J (2012) Angiogenesis impairment in diabetes: role of methylglyoxal-induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS One 7:e46720
Bachar-Wikstrom E, Wikstrom JD, Ariav Y et al (2013) Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes 62:1227–1237
Bartolome A, Guillen C, Benito M (2012) Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic beta cell death. Autophagy 8:1757–1768
Xie Z, Lau K, Eby B et al (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60:1770–1778
Tanaka Y, Kume S, Kitada M et al (2012) Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res: 628978
Shimizu K, Kiuchi Y, Ando K, Hayakawa M, Kikugawa K (2004) Coordination of oxidized protein hydrolase and the proteasome in the clearance of cytotoxic denatured proteins. Biochem Biophys Res Commun 324:140–146
Venkatesh S, Lee J, Singh K, Lee I, Suzuki CK (2012) Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim Biophys Acta 1823:56–66
Padrao AI, Carvalho T, Vitorino R et al (2012) Impaired protein quality control system underlies mitochondrial dysfunction in skeletal muscle of streptozotocin-induced diabetic rats. Biochim Biophys Acta 1822:1189–1197
Lee HJ, Chung K, Lee H, Lee K, Lim JH, Song J (2011) Downregulation of mitochondrial lon protease impairs mitochondrial function and causes hepatic insulin resistance in human liver SK-HEP-1 cells. Diabetologia 54:1437–1446
Niforou K, Cheimonidou C, Trougakos IP (2014) Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol 2:323–332
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451
Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S (2009) Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci 10:85–95
Chien V, Aitken JF, Zhang S et al (2010) The chaperone proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and formation of beta-sheet-containing aggregates by human amylin: a potential role for defective chaperone biology in type 2 diabetes. Biochem J 432:113–121
Chan JY, Luzuriaga J, Bensellam M, Biden TJ, Laybutt DR (2013) Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes 62:1557–1568
Chung J, Nguyen AK, Henstridge DC et al (2008) HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A 105:1739–1744
Henstridge DC, Bruce CR, Drew BG et al (2014) Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. doi:10.2337/db13-0967
Bruce CR, Carey AL, Hawley JA, Febbraio MA (2003) Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52:2338–2345
Kurucz I, Morva A, Vaag A et al (2002) Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51:1102–1109
Hooper PL (2009) Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones 14:113–115
Atalay M, Oksala NK, Laaksonen DE et al (2004) Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 97:605–611
Barutta F, Pinach S, Giunti S et al (2008) Heat shock protein expression in diabetic nephropathy. Am J Physiol Renal Physiol 295:F1817–F1824
Literati-Nagy B, Tory K, Peitl B et al (2014) Improvement of insulin sensitivity by a novel drug candidate, BGP-15, in different animal studies. Metab Syndr Relat Disord 12:125–131
Ma J, Farmer KL, Pan P et al (2014) Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J Pharmacol Exp Ther 348:281–292
McCarty MF (2006) Induction of heat shock proteins may combat insulin resistance. Med Hypotheses 66:527–534
Nanasi PP, Jednakovits A (2001) Multilateral in vivo and in vitro protective effects of the novel heat shock protein coinducer, bimoclomol: results of preclinical studies. Cardiovasc Drug Rev 19:133–151
Vigh L, Literati PN, Horvath I et al (1997) Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med 3:1150–1154
Zhang L, Zhao H, Blagg BS, Dobrowsky RT (2012) C-terminal heat shock protein 90 inhibitor decreases hyperglycemia-induced oxidative stress and improves mitochondrial bioenergetics in sensory neurons. J Proteome Res 11:2581–2593
Kurthy M, Mogyorosi T, Nagy K et al (2002) Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann N Y Acad Sci 967:482–489
Ziegler D, Nowak H, Kempler P, Vargha P, Low PA (2004) Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med 21:114–121
Evans JL, Goldfine ID (2000) Alpha-lipoic acid: a multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol Ther 2:401–413
Davis BJ, Xie Z, Viollet B, Zou MH (2006) Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55:496–505
Van Schaftingen E, Collard F, Wiame E, Veiga-da-Cunha M (2012) Enzymatic repair of Amadori products. Amino Acids 42:1143–1150
Kim HY, Gladyshev VN (2007) Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J 407:321–329
Mary J, Vougier S, Picot CR, Perichon M, Petropoulos I, Friguet B (2004) Enzymatic reactions involved in the repair of oxidized proteins. Exp Gerontol 39:1117–1123
Clarke S (2003) Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev 2:263–285
Shimizu T, Matsuoka Y, Shirasawa T (2005) Biological significance of isoaspartate and its repair system. Biol Pharm Bull 28:1590–1596
Delpierre G, Rider MH, Collard F et al (2000) Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase. Diabetes 49:1627–1634
Collard F, Wiame E, Bergans N et al (2004) Fructosamine 3-kinase-related protein and deglycation in human erythrocytes. Biochem J 382:137–143
Li Y, Zhang W, Li P, Huang K (2011) Effect of streptozocin-induced diabetes mellitus on expression of methionine sulfoxide reductases and accumulation of their substrates in mouse lenses. Exp Eye Res 92:401–407
Wagner AM, Cloos P, Bergholdt R et al (2007) Post-translational protein modifications in type 1 diabetes: a role for the repair enzyme protein-l-isoaspartate (d-aspartate) O-methyltransferase? Diabetologia 50:676–681
Wagner AM, Cloos P, Bergholdt R et al (2008) Posttranslational protein modifications in type 1 diabetes – genetic studies with PCMT1, the repair enzyme protein isoaspartate methyltransferase (PIMT) encoding gene. Rev Diabet Stud 5:225–231
Delpierre G, Veiga-da-Cunha M, Vertommen D, Buysschaert M, van Schaftingen E (2006) Variability in erythrocyte fructosamine 3-kinase activity in humans correlates with polymorphisms in the FN3K gene and impacts on haemoglobin glycation at specific sites. Diabetes Metab 32:31–39
Pascal SM, Veiga-da-Cunha M, Gilon P, van Schaftingen E, Jonas JC (2010) Effects of fructosamine-3-kinase deficiency on function and survival of mouse pancreatic islets after prolonged culture in high glucose or ribose concentrations. Am J Physiol Endocrinol Metab 298:E586–E596
Mosca L, Penco S, Patrosso MC et al (2011) Genetic variability of the fructosamine 3-kinase gene in diabetic patients. Clin Chem Lab Med 49:803–808
Tanhauserova V, Kuricova K, Pacal L et al (2014) Genetic variability in enzymes of metabolic pathways conferring protection against non-enzymatic glycation versus diabetes-related morbidity and mortality. Clin Chem Lab Med 52:77–83
Acknowledgements
The authors would like to thank E. van Schaftingen (de Duve Institute, Brussels, Belgium) for carefully reading the manuscript and providing constructive comments.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
Both authors were responsible for the conception and design of the manuscript, drafting the article and revising it critically for important intellectual content. Both authors approved the version to be published.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaisson, S., Gillery, P. Impaired proteostasis: role in the pathogenesis of diabetes mellitus. Diabetologia 57, 1517–1527 (2014). https://doi.org/10.1007/s00125-014-3257-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3257-1