Abstract
Aims/hypothesis
Hypoglycaemia in association with breastfeeding is a feared condition in mothers with type 1 diabetes. Thus, routine carbohydrate intake at each breastfeed, particularly at night, is often recommended despite lack of evidence. We aimed to evaluate glucose levels during breastfeeding, focusing on whether night-time breastfeeding induced hypoglycaemia in mothers with type 1 diabetes.
Methods
Of 43 consecutive mothers with type 1 diabetes, 33 (77%) were included prospectively 1 month after a singleton delivery. Twenty-six mothers (mean [SD] age 30.7 [5.8] years, mean [SD] duration of diabetes 18.6 [10.3] years) were breastfeeding and seven mothers (mean [SD] age 31.7 [5.6] years, mean [SD] duration of diabetes 20.4 [6.2] years) were bottle-feeding their infants with formula. All were experienced in carbohydrate counting using individually tailored insulin therapy with insulin analogues (45% on insulin pump, 55% on multiple daily injections). Thirty-two women with type 1 diabetes, matched for age ±1 year and BMI ±1 kg/m2, who had not given birth or breastfed in the previous year, served as a control group. Blinded continuous glucose monitoring (CGM) for 6 days was applied at 1, 2 and 6 months postpartum in the breastfeeding mothers who recorded breastfeeds and carbohydrate intake at each CGM period. CGM was applied at 1 month postpartum in the formula-feeding mothers and once in the control women. The insulin dose was individually tailored after each CGM period.
Results
The percentage of night-time spent with CGM <4.0 mmol/l was low (4.6%, 3.1% and 2.7% at each CGM period in the breastfeeding mothers vs 1.6% in the control women, p = 0.77), and the breastfeeding mothers spent a greater proportion of the night-time in the target range of 4.0–10.0 mmol/l (p = 0.01). Symptomatic hypoglycaemia occurred two or three times per week at 1, 2 and 6 months postpartum in both breastfeeding mothers and the control women. Severe hypoglycaemia was reported by one mother (3%) during the 6 month postpartum period and by one control woman (3%) in the previous year (p = 0.74). In breastfeeding mothers at 1 month, the insulin dose was 18% (−67% to +48%) lower than before pregnancy (p = 0.04). In total, carbohydrate was not consumed in relation to 438 recorded night-time breastfeeds, and CGM <4.0 mmol/l within 3 h occurred after 20 (4.6%) of these breastfeeds.
Conclusions/interpretation
The percentage of night-time spent in hypoglycaemia was low in the breastfeeding mothers with type 1 diabetes and was similar in the control women. Breastfeeding at night-time rarely induced hypoglycaemia. The historical recommendation of routine carbohydrate intake at night-time breastfeeding may be obsolete in mothers with type 1 diabetes who have properly reduced insulin dose with sufficient carbohydrate intake.
Trial registration
ClinicalTrials.gov NCT02898428
Similar content being viewed by others

Introduction
The World Health Organization recommends exclusive breastfeeding up to 6 months of age [1] to reduce the offspring’s risk of future obesity [2,3,4,5], type 1 diabetes [6,7,8] and type 2 diabetes [9, 10]. In breastfeeding mothers with normal glucose tolerance, suckling does not affect glucose levels as documented by continuous glucose monitoring (CGM) [11, 12]. Historically, concerns that mothers with type 1 diabetes may experience hypoglycaemia soon after they breastfeed [13, 14] led to them being advised to eat before or during each breastfeeding [15]. Reducing the insulin dose by up to 27% compared with the pre-pregnancy dose has been suggested to prevent hypoglycaemia during breastfeeding [16, 17]. In 12 breastfeeding mothers with type 1 diabetes treated via insulin pump, basal insulin doses were lower during the 2 month period postpartum while the risk of hypoglycaemia was high during the first 2 weeks postpartum, although carbohydrate intake was not recorded [18].
Recently, a study of blinded CGM (i.e. the study participants could not see the measurements) applied for 6 days during breastfeeding in eight mothers with type 1 diabetes showed that the mothers spent 38% of CGM at night in hypoglycaemia (glucose levels <4.0 mmol/l) but only 9% of total CGM time in hypoglycaemia. The insulin dose during breastfeeding was at the same level as before pregnancy [19].
In the greater Copenhagen area, women with type 1 diabetes are trained to use carbohydrate counting and flexible insulin treatment with insulin analogues in either insulin pumps or basal bolus therapy.
At the maternity unit at Rigshospitalet, mothers with type 1 diabetes have for years been instructed routinely to consume 10–20 g of carbohydrate at night-time breastfeeds to avoid subsequent hypoglycaemia [20]. Besides the inconvenience of eating during the night, there is no solid evidence that this practice is necessary to obtain stable glucose levels without night-time hypoglycaemia among breastfeeding women with type 1 diabetes on flexible insulin therapy when an appropriate reduction of scheduled insulin dose and a sufficient daily carbohydrate intake are recommended postpartum.
We aimed to evaluate glucose levels during breastfeeding with intermittent CGM and focus on hypoglycaemia at night and whether night-time breastfeeding induced hypoglycaemia within 3 h of breastfeeding in mothers with type 1 diabetes on flexible insulin therapy.
Methods
Participants
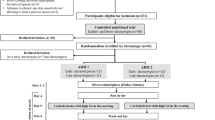
This prospective cohort study included Danish-speaking consecutive mothers with type 1 diabetes who delivered a single infant at the Department of Obstetrics, Rigshospitalet from 17 April 2016 to 22 October 2017 and who attended diabetes control at Steno Diabetes Center Copenhagen during pregnancy and within 3 months postpartum. This population was regarded as a random sample from the referral area.
To compare the levels of hypoglycaemia during CGM, 32 control women with type 1 diabetes, matched for age ±1 year and BMI ±1 kg/m2, who in the preceding year had not given birth or breastfed, were identified from the electronic patient records at Steno Diabetes Center Copenhagen. All control women had a negative pregnancy test on the day of inclusion in the study.
Exclusion criteria were psychosocial barriers or a concurrent disease.
The study participants gave written informed consent to participate. The research protocol was approved by the Regional Committee on Biomedical Research Ethics (Protocol number H-16016397) and the Danish Data Protection Agency (Protocol number 2012-58-0004) and was carried out in accordance with the Declaration of Helsinki (2008). The study was registered with ClinicalTrials.gov (registration no. NCT02898428).
CGM
Blinded CGM (iPro2 Professional CGM; Medtronic, Northridge, CA, USA) was applied for 6 days (mainly by L. Ringholm) to the mothers at 1 month (mean [SD] 36 [11] days), 2 months (68 [14] days) and 6 months (177 [13] days) postpartum and to the control women for one 6 day period. The CGM device was inserted according to the manufacturer’s guidelines into the subcutaneous tissue of the abdominal skin. All women were encouraged to continue normal daily living during each CGM period without changing breastfeeding, food or exercise patterns.
Finger prick capillary blood glucose levels were measured with a Contour Next glucometer (Ascensia Diabetes Care Denmark, Copenhagen, Denmark) 1 and 3 h after insertion. All women were recommended to perform self-monitoring of plasma glucose (SMPG) at least four times daily, before main meals and before bedtime. The target SMPG range was 4.0–10.0 mmol/l.
Twelve mothers and four control women used CGM routinely. Of these, three agreed to use the Ipro CGM device for this study. Regardless of type of routine CGM device, the CGM data from the remaining 13 women were used in this study (Table 1).
Night-time was defined as 23:00 to 06:59 h and daytime was defined as 07:00 to 22:59 h.
Diabetes management
All women were recommended an individualised diabetes diet based on the national Danish recommendations for individuals with diabetes. The breastfeeding mothers were recommended a minimum daily total carbohydrate intake of 210 g in accordance with the Institute of Medicine (IOM) guidelines to prevent ketonaemia (www.nap.edu/read/10490/chapter/8#293, accessed 31 July 2018). All were recommended to count carbohydrates from the main carbohydrate sources (bread, potatoes, rice, pasta, fruits, dairy products and confectionary) at all meals and snacks. The amount of carbohydrates from the remaining sources in a diabetes diet was judged to be 25 g daily, resulting in a recommended minimum daily intake of 185 g from main sources in breastfeeding mothers.
Based on the local treatment protocol, the insulin dose immediately postpartum was individually tailored by an endocrinologist and was intended to be approximately 60% of the pre-pregnancy dose. This involved appropriate changes in basal insulin, carbohydrate:insulin ratio and insulin sensitivity, resulting in approximately 40% reductions in basal, meal and correction insulin doses.
The insulin dose was adjusted after each CGM period, based on CGM data in combination with SMPG values, focusing on obtaining most time in the target range of 4.0–10.0 mmol/l while avoiding hypoglycaemia.
All women were recommended to do at least 30 min of exercise daily.
Breastfeeding, food and exercise diary
During each 6 day CGM period the mothers were encouraged to fill in a food diary, which is used as part of routine care in our centre [21]. For this study, the diary was expanded to include breastfeeding and exercise. Each breastfeed was noted, with information on time of onset and whether an extra snack due to breastfeeding was consumed. The control women recorded a food and exercise diary during the 6 day CGM period. All women recorded self-estimated carbohydrate content from the main carbohydrate sources at all meals and snacks, as well as type and duration of exercise.
The quantity of self-estimated carbohydrate intake from the main carbohydrate sources was calculated. When in doubt, the registered dietitian (A. B. Roskjær) estimated the dimensions, weight and portion sizes based on validated tables (www.vitakost.dk/en/home, accessed 31 July 2018, www.food.dtu.dk/english/-/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2013/Rapport_Maal-vaegt-og-portionsstoerrelser-paa-foedevarer.ashx?la=da, accessed 31 July 2018).
Evaluation of CGM data
After each CGM period, each woman discussed the downloaded CGM data with an endocrinologist (L. Ringholm or S. Engberg). The primary focus was glycaemic trends during night-time, with emphasis on the prevention of hypoglycaemia. Thereafter, hypoglycaemia and pre- and postprandial glucose values during daytime were evaluated, with focus on average daily total carbohydrate intake and day-to-day variation, aiming for glucose values in the target range of 4.0–10.0 mmol/l.
The following CGM data were recorded at night-time and over 24 h: mean glucose values; percentage of time spent with glucose values in the target range (4.0–10.0 mmol/l); percentage of time spent in hypoglycaemia (<4.0 mmol/l) and percentage of time spent in hyperglycaemia (>10.0 mmol/l).
Mild hypoglycaemia
Mild hypoglycaemia was defined as events with symptoms familiar to the woman as hypoglycaemia and managed by her [22].
Severe hypoglycaemia
Severe hypoglycaemia was defined as events requiring assistance from others to restore normal glucose levels [23]. Where severe hypoglycaemia occurred in the period from delivery until 6 months postpartum, we performed a structured interview previously used in prospective studies among pregnant [24, 25] and non-pregnant [22] women with type 1 diabetes. The interview was modified to also address breastfeeding.
If the control women reported severe hypoglycaemia in the previous year, a structured interview was performed on the day of inclusion in the study.
Hypoglycaemia awareness
Self-estimated hypoglycaemia awareness was derived from the woman’s answer to the question: ‘How often do you recognise symptoms, when you have a hypo?’ [22, 26]. Women answering ‘always’ were classified as having normal awareness, those answering ‘usually’ were classified as having impaired awareness and those answering ‘occasionally’ or ‘never’ were classified as having unawareness.
Questionnaires
At 1, 2 and 6 months, the mothers filled in a questionnaire slightly modified from [27], focusing on breastfeeding and hypoglycaemia. The questions encompassed number of daily breastfeed sessions in the previous week, number of daily formula feedings in the previous week, mild hypoglycaemia in the previous week, severe hypoglycaemia since delivery, hypoglycaemia awareness status and smoking status. Sociodemographic data were collected in the questionnaire at inclusion.
The control women filled in a questionnaire on mild hypoglycaemia in the previous week, severe hypoglycaemia in the previous year, hypoglycaemia awareness status, smoking status and sociodemographic data.
Education level was classified in accordance with the International Standard Classification of Education and converted to three educational levels: ≤10 years, 11–14 years and ≥15 years of education (uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf, accessed 31 July 2018).
Full or predominant breastfeeding was defined as six or more breastfeeding sessions daily [19]. Gestational weight gain was calculated as the difference between the last weight measured before delivery and the self-reported pre-pregnancy weight [28]. Gestational weight gain retention was defined as >5.0 kg compared with pre-pregnancy weight [29].
At 1 month postpartum, the mothers gave information on offspring gestational age, weight and length at delivery. Information on admittance to a neonatal intensive care unit was taken from the records.
On the days of application of CGM, HbA1c was measured by a TOSOH G8 Automated Glycohemoglobin Analyzer (Tosoh Corporation, Tokyo, Japan).
Small- and large-for-gestational-age infants were defined as offspring birthweight ≤10th or ≥90th percentile, respectively, adjusted for sex and gestational age [30].
Sample size
Although breastfeeding mothers have been reported to spend 38% of night-time in hypoglycaemia [19], we conservatively assumed that in our population the time spent in night-time hypoglycaemia was lower (i.e. mean 18% [variance 9%] in breastfeeding mothers and mean 6% [variance 3%] in control women). Thus, these figures were used when calculating sample size. We assumed that 50% of all mothers breastfed at 2 months. A sample size of 15 or more breastfeeding mothers implied that the power of the test was well over 80% and would protect against departures from underlying assumptions of normality. However, deviation from assumed prevalence in outcome variables is common. Therefore, we included all consecutive mothers during a 1.5 year period aiming to include more than 15 mothers who breastfed at 2 months.
Statistical analyses
Normally distributed data were reported as mean (SD), non-normally distributed data as median (range) and categorical data as n (%). All comparisons were with the breastfeeding mothers.
Continuous variables were compared by paired t test or non-parametric tests when appropriate. Categorical variables were compared by χ2 test or Fisher’s exact test, as appropriate.
Repeated measurements were analysed by a mixed model with participant identification as random effect to take intra-individual measurement correlation into account.
To compare repeated CGM data in the breastfeeding mothers with the CGM data in the control women, a mixed model was used with random effect of matched pairs (breastfeeding mothers and matched control women).
A two-sided p value <0.05 was regarded as statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
In the study period, 48 mothers with type 1 diabetes delivered. Two mothers did not attend diabetes control within 3 months postpartum and three mothers were not approached for the study due to psychosocial barriers, leaving 43 mothers of which 33 (77%) agreed to participate in the study. CGM data were available in all 33 mothers at 1 month postpartum.
At 1 month, 26 of the 33 (79%) mothers were breastfeeding their infants and seven mothers were formula feeding. One mother discontinued breastfeeding at 1–2 months and another seven mothers discontinued breastfeeding at 2–6 months postpartum (Tables 2 and 3).
The 33 mothers and the 32 control women had comparable age, BMI, duration of diabetes and use of insulin pumps, while routine use of CGM was more prevalent in the mothers than in the control women (Table 1). Mean educational level was lower in breastfeeding mothers than in the control women (15.8 [2.6] vs 17.0 [2.1] years, p = 0.03), although similar percentages of breastfeeding mothers and control women had educational level ≥15 years (77% vs 84%, p = 0.52) (Table 1). HbA1c levels were lower in the breastfeeding mothers than in the control women (Table 2).
Immediately postpartum, the actual recommended daily insulin dose (rapid- and long-acting insulin analogues) was 24% (−6% to −54%) lower than before pregnancy in the mothers on multiple daily injections while basal insulin dose was 29% (−19% to −39%) lower than before pregnancy in mothers on insulin pump therapy. In breastfeeding mothers the insulin dose at 1 month was 18% (−67% to +48%) lower than before pregnancy (p = 0.04). At 2 and 6 months postpartum, breastfeeding mothers’ insulin dose was 14% (−54% to +57%) and 4% (−59% to +41%) lower than before pregnancy (p = 0.08 and p = 0.90), respectively.
The daily carbohydrate intake from the main sources was stable at 169–182 g among breastfeeding mothers from 1 month to 6 months postpartum (i.e. 11–24 g more than in the control women) (Table 2).
The percentage of night-time spent with CGM <4.0 mmol/l was low (4.6%, 3.1% and 2.7% at each CGM period in the breastfeeding mothers vs 1.6% in the control women, p = 0.77) (Table 4). The breastfeeding mothers spent more time in the target range 4.0–10.0 mmol/l and less time with CGM >10.0 mmol/l and had lower mean glucose levels compared with the control women (Table 4).
At 1, 2 and 6 months, the percentage of night-time that the breastfeeding mothers spent in the target range and with CGM <4.0 mmol/l remained stable (p = 0.29 and p = 0.96, respectively) (Table 4). The same was seen in the subgroup of mothers breastfeeding for at least 6 months (p = 0.71 and p = 0.64, respectively) (Table 5).
The time spent in target range and with CGM <4.0 mmol/l were comparable between breastfeeding mothers and control women using routine CGM and/or insulin pump (Table 6).
Breastfeeding, food and exercise diaries were available for 21 breastfeeding mothers who filled in the diaries at three (one to three) CGM periods. Combining breastfeeding data from all three CGM periods, a total of 471 night-time breastfeeds were recorded. CGM <4.0 mmol/l within 3 h after night-time breastfeeding occurred after 32 (6.8%) of all night-time breastfeeds in 15 mothers, of whom eight (53%) reported normal hypoglycaemia awareness. At 1, 2 and 6 months, there were 11, 10 and 11 episodes with CGM <4.0 mmol/l, respectively.
Carbohydrates were not consumed in association with the majority (93%) of breastfeeds at night. Among 438 night-time breastfeeds without carbohydrate intake, 20 (4.6%) were followed by CGM <4.0 mmol/l within the following 3 h in 12 mothers, of whom five (42%) reported normal hypoglycaemia awareness.
A total of 1237 day-time breastfeeds were recorded. CGM <4.0 mmol/l within 3 h after daytime breastfeeding occurred after 72 (5.8%) of all daytime breastfeeds in 18 mothers of whom 11 (61%) reported normal hypoglycaemia awareness. At 1, 2 and 6 months, there were 28, 20 and 24 episodes with CGM <4.0 mmol/l, respectively.
The glucose levels at the beginning of breastfeedings or the timing of breastfeeds were not related to CGM <4.0 mmol/l within 3 h after breastfeeding during night- or daytime.
In the breastfeeding mothers, the number of weekly episodes of mild hypoglycaemia was lower at 2 months compared with 1 month, but this did not reach statistical significance (p = 0.06) (Table 2). The number of weekly episodes of mild hypoglycaemia and the proportions with normal hypoglycaemia awareness were similar in the breastfeeding mothers and in the control women (Table 2).
Severe hypoglycaemia was reported by one (3%) mother and one (3%) control woman (p = 0.74). The mother reported two episodes of severe hypoglycaemia within 2–4 weeks postpartum, during a period when she was manually expressing breast milk. The control woman reported one episode of severe hypoglycaemia within the previous year.
The majority of the breastfeeding mothers had no gestational weight gain retention from 2 months onwards (Table 2). Among the breastfeeding mothers at 2 months, gestational weight gain was 13.1 (3.6) kg in the 21 mothers without gestational weight gain retention and 19.2 (2.8) kg in the five mothers with gestational weight gain retention (p = 0.004).
No incidences of ketoacidosis were reported. Five mothers were on antihypertensive therapy, of which two received labetalol for 1–2 months postpartum. Excluding women who smoked did not change the results (data not shown).
Discussion
This prospective study with intermittent use of CGM postpartum demonstrated that breastfeeding mothers with type 1 diabetes spent a greater percentage of the night-time in the target range compared with control women, while the percentage of night-time spent with CGM <4.0 mmol/l was similar when comparing breastfeeding mothers with control women.
At the majority of night-time breastfeeds, carbohydrate was not consumed and still night-time breastfeeding was rarely followed by CGM <4.0 mmol/l within the next 3 h. This is in agreement with previous observations in eight mothers with type 1 diabetes where glucose remained over 4.0 mmol/l after the majority of suckling episodes [19] and in mothers with normal glucose tolerance where suckling did not affect glucose profiles whether or not the mothers were fasting [11, 12].
The insulin dose was reduced and specifically tailored in each mother upon delivery and then further tailored after each CGM period as indicated. The daily carbohydrate intake and amount of exercise in breastfeeding mothers were remarkably stable from 1 month to 6 months postpartum. The breastfeeding mothers consumed approximately 180 g of carbohydrate from the main sources, corresponding to 205 g in total, which is close to the recommended minimum daily total carbohydrate intake of 210 g in the IOM guidelines (www.nap.edu/read/10490/chapter/8#293, accessed 31 July 2018). The carbohydrate intake was 11–24 g higher in the breastfeeding mothers than in the control women. This intensive focus on appropriate insulin dose reduction during breastfeeding and sufficient carbohydrate intake probably contributed to the low percentage of time spent in hypoglycaemia.
The daily carbohydrate intake of around 180 g was lower than in another study [19] where breastfeeding mothers consumed 237 g of carbohydrate daily; this may partly be explained by the high night-time hypoglycaemia prevalence of 38%.
Immediately postpartum, the insulin dose was reduced by approximately 24% compared with the pre-pregnancy dose. At 1 month, the insulin dose in breastfeeding mothers remained 18% lower than the pre-pregnancy dose. Insulin requirements increased gradually over the following months. Lessons learnt from this study are that the endocrinologist did not reduce the insulin dose as much as the protocol recommended and the breastfeeding mothers increased their insulin dose between visits. However, the 18% reduction in insulin dose at 2 months is in accordance with our previous data [17] where the insulin dose was 21% lower at 2–4 months compared with pre-pregnancy dose in 105 breastfeeding mothers with type 1 diabetes. Based on these observations, we are changing our protocol so that the insulin dose immediately postpartum will be individually tailored to approximately 70% of the pre-pregnancy dose.
Mild hypoglycaemia occurred two or three times per week and the incidence of severe hypoglycaemia was low in breastfeeding mothers and control women, in agreement with previous findings at our centre [17]. The proportion of breastfeeding mothers with normal hypoglycaemia awareness remained stable at around 70% during the study, similar to the control women. However, only half of the mothers with CGM <4.0 mmol/l within 3 h after breastfeeding reported normal hypoglycaemia awareness.
The majority of the mothers were trained in using carbohydrate counting and flexible insulin therapy, therefore they were accustomed to assessing insulin dose at carbohydrate intake postpartum with an appropriately adjusted carbohydrate:insulin ratio. All the mothers were on insulin analogues, almost half were on insulin pump therapy and one-third used CGM routinely. This may also have contributed to the low percentage of time spent in hypoglycaemia.
Breastfeeding mothers had better glycaemic control than control women, as judged by mean glucose levels and the amount of time spent in the target range, despite higher carbohydrate intake and lower insulin dose. In particular, the 16 mothers who breastfed long-term and the women using diabetes technology tended to have slightly better CGM profiles with less hypoglycaemia, although the numbers were too small for solid conclusions.
Glycaemic control and risk of hypoglycaemia may change during the 6 months of breastfeeding. We therefore included three CGM periods in this study whereas glycaemic control in the control women was expected to be stable. The time spent in target range and in hypoglycaemia were comparable at 1, 2 and 6 months, suggesting that the physiological changes during this span of breastfeeding were of lesser importance for glycaemic control.
At 2 months postpartum, the majority of the breastfeeding mothers were already within 5.0 kg of their pre-pregnancy weight. Exclusive breastfeeding increases energy consumption by 1300–1900 kJ/day in the first 6 months postpartum [18, 31]. Other factors contributing to achieving pre-pregnancy body weight include appropriate gestational weight gain, focus on quantity and quality of carbohydrate intake and total energy intake [32]. Sufficient reduction in insulin dose postpartum, limiting the need for carbohydrate intake to avoid hypoglycaemia, may also be important.
Low-carbohydrate diets are popular in healthy persons [33] and in individuals with type 1 diabetes. However, breastfeeding mothers with type 1 diabetes need to consume sufficient carbohydrates to secure sufficient milk production and to avoid ketonaemia and hypoglycaemia while breastfeeding [34]. A minimum daily total carbohydrate intake of 210 g has been suggested during breastfeeding (www.nap.edu/read/10490/chapter/8#293, accessed 31 July 2018). A daily intake of 180 g carbohydrate from the main sources, as in the current study, corresponds roughly to 205 g carbohydrate by adding approximately 25 g from vegetables and other minor sources [35]. We did not investigate whether ketonaemia occurred but there were no incidences of ketoacidosis. Starvation ketoacidosis has been reported in breastfeeding mothers without diabetes [33, 36, 37].
Our data suggest that tight glycaemic control can be maintained during breastfeeding and that glucose targets similar to those for non-breastfeeding women with type 1 diabetes can be recommended in breastfeeding mothers with type 1 diabetes using flexible insulin therapy with an appropriate insulin dose reduction immediately postpartum and sufficient carbohydrate intake.
Strengths of this study include the high participation rate (77% of eligible mothers with type 1 diabetes participating), similar to studies during pregnancy [25, 38] and higher than in a previous study investigating the first 4 weeks postpartum [39]. The mothers were matched for age and pre-pregnancy BMI with control women. The high attendance rate of unselected mothers with diabetes who recently gave birth suggests that these data are generalisable to breastfeeding mothers with diabetes, subject to early insulin dose reduction and appropriate carbohydrate intake. However, we cannot rule out that our study findings can be generalised to mothers who are not adherent to treatment regimen or not receptive to careful management practices.
The observed percentages of night-time spent in hypoglycaemia (4.6%, 3.1% and 2.7% at 1, 2 and 6 months postpartum, respectively) among breastfeeding mothers were close to those seen in the control women, and much lower than the 38% previously reported in a study where the recommended insulin dose postpartum was not reduced appropriately and recommendations regarding daily carbohydrate intake were not reported [19]. A limitation of our study is that a considerably higher number of women would be needed to evaluate whether the observed small difference between breastfeeding mothers and control women reflected a statistically significant difference. However, the observed percentages of night-time spent in hypoglycaemia are at a clinically acceptable low level and the observed difference between breastfeeding mothers and control women is probably not of clinical importance.
Educational level was lower both in the breastfeeding mothers and in all 33 mothers compared with control women. Smoking was not prevalent and correction for smoking did not affect results. The high prevalence of breastfeeding and thereby low number of formula-feeding mothers limited the possibility for subanalyses. A randomised study on breastfeeding would not be ethical. CGM data in the very early postpartum period, where hypoglycaemia may be prevalent [39], were not available.
In summary, the percentage of night-time spent in hypoglycaemia was low and similar in the breastfeeding mothers with type 1 diabetes and in control women. Breastfeeding at night-time rarely induced hypoglycaemia. The established recommendation of routine carbohydrate intake at night-time breastfeeding may be obsolete in mothers with type 1 diabetes who have properly reduced insulin dose and who have sufficient carbohydrate intake.
Abbreviations
- CGM:
-
Continuous glucose monitoring
- IOM:
-
Institute of Medicine
- SMPG:
-
Self-monitoring of plasma glucose
References
(1990) Protecting, promoting and supporting breastfeeding: the special role of maternity services. A joint WHO/UNICEF statement. Int J Gynaecol Obstet 31(Suppl 1):171–183
Bergmann KE, Bergmann RL, Von KR et al (2003) Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord 27(2):162–172. https://doi.org/10.1038/sj.ijo.802200
Feig DS, Lipscombe LL, Tomlinson G, Blumer I (2011) Breastfeeding predicts the risk of childhood obesity in a multi-ethnic cohort of women with diabetes. J Matern Fetal Neonatal Med 24(3):511–515. https://doi.org/10.3109/14767058.2010.500711
Hummel S, Pfluger M, Kreichauf S, Hummel M, Ziegler AG (2009) Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care 32(5):921–925. https://doi.org/10.2337/dc08-1943
Weyermann M, Rothenbacher D, Brenner H (2006) Duration of breastfeeding and risk of overweight in childhood: a prospective birth cohort study from Germany. Int J Obes 30(8):1281–1287. https://doi.org/10.1038/sj.ijo.0803260
Borch-Johnsen K, Joner G, Mandrup-Poulsen T et al (1984) Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. A hypothesis. Lancet 2:1083–1086
Kimpimaki T, Erkkola M, Korhonen S et al (2001) Short-term exclusive breastfeeding predisposes young children with increased genetic risk of type I diabetes to progressive beta cell autoimmunity. Diabetologia 44(1):63–69. https://doi.org/10.1007/s001250051581
Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Z, Jasinskiene E, Samuelsson U (2004) Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev 20(2):150–157. https://doi.org/10.1002/dmrr.425
Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG (2006) Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 84(5):1043–1054. https://doi.org/10.1093/ajcn/84.5.1043
Pettitt DJ, Forman MR, Hanson RL, Knowler WC, Bennett PH (1997) Breastfeeding and incidence of non-insulin-dependent diabetes mellitus in Pima Indians. Lancet 350(9072):166–168. https://doi.org/10.1016/S0140-6736(96)12103-6
Bentley-Lewis R, Goldfine AB, Green DE, Seely EW (2007) Lactation after normal pregnancy is not associated with blood glucose fluctuations. Diabetes Care 30(11):2792–2793. https://doi.org/10.2337/dc07-1243
Colatrella A, Framarino M, Toscano V et al (2012) Continuous glucose monitoring during breastfeeding in women with recent gestational diabetes mellitus. Diabetes Technol Ther 14(7):576–582. https://doi.org/10.1089/dia.2011.0266
Neubauer SH (1990) Lactation in insulin-dependent diabetes. Prog Food Nutr Sci 14:333–370
Stubblefield NE (1988) Nutrition management of diabetes during pregnancy. Top Clin Nutr 3(1):58–63. https://doi.org/10.1097/00008486-198801000-00010
McLaughlin C, McCance DR (2010) Diabetic management in labor, delivery and post delivery. In: McCance DR, Maresh M, Sacks DA (eds) A practical manual of diabetes in pregnancy, 1st Edition edn. Wiley-Blackwell, Chichester, pp 211–219. https://doi.org/10.1002/9781444315196.ch21
Davies HA, Clark JD, Dalton KJ, Edwards OM (1989) Insulin requirements of diabetic women who breast feed. BMJ 298(6684):1357–1358. https://doi.org/10.1136/bmj.298.6684.1357
Herskin CW, Stage E, Barfred C et al (2015) Low prevalence of long-term breastfeeding among women with type 2 diabetes. J Matern Fetal Neonatal Med 29:2512–2517
Riviello C, Mello G, Jovanovic LG (2009) Breastfeeding and the basal insulin requirement in type 1 diabetic women. Endocr Prac 15(3):187–193. https://doi.org/10.4158/EP.15.3.187
Achong N, McIntyre HD, Callaway L, Duncan EL (2016) Glycaemic behaviour during breastfeeding in women with type 1 diabetes. Diabet Med 33(7):947–955. https://doi.org/10.1111/dme.12993
Ringholm L, Ásbjörnsdóttir B, Andersen HU, Damm P, Mathiesen ER (2018) Dietary advice and glycaemic control in women with type 1 diabetes during preconception counselling, pregnancy and breastfeeding. In: Rajendram R, Preedy V, Patel VB (eds) Nutrition and diet in maternal diabetes, 1st edn. Springer Nature, Cham, pp 385–397. https://doi.org/10.1007/978-3-319-56440-1_30
Ásbjörnsdóttir B, Akueson CE, Ronneby H et al (2017) The influence of carbohydrate consumption on glycemic control in pregnant women with type 1 diabetes. Diabetes Res Clin Pract 127:97–104. https://doi.org/10.1016/j.diabres.2016.12.012
Pedersen-Bjergaard U, Pramming S, Thorsteinsson B (2003) Recall of severe hypoglycaemia and self-estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev 19(3):232–240. https://doi.org/10.1002/dmrr.377
Seaquist ER, Anderson J, Childs B et al (2013) Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 36(5):1384–1395. https://doi.org/10.2337/dc12-2480
Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER (2008) Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care 31(1):9–14. https://doi.org/10.2337/dc07-1066
Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER (2013) The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 36(7):1877–1883. https://doi.org/10.2337/dc12-2360
Hoi-Hansen T, Pedersen-Bjergaard U, Thorsteinsson B (2010) Classification of hypoglycemia awareness in people with type 1 diabetes in clinical practice. J Diabetes Complicat 24(6):392–397. https://doi.org/10.1016/j.jdiacomp.2009.07.006
Stage E, Norgard H, Damm P, Mathiesen E (2006) Long-term breast-feeding in women with type 1 diabetes. Diabetes Care 29(4):771–774. https://doi.org/10.2337/diacare.29.04.06.dc05-1103
Jensen DM, Damm P, Sorensen B et al (2003) Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am J Obstet Gynecol 189(1):239–244. https://doi.org/10.1067/mob.2003.441
Vinter CA, Jensen DM, Ovesen P et al (2014) Postpartum weight retention and breastfeeding among obese women from the randomized controlled Lifestyle in Pregnancy (LiP) trial. Acta Obstet Gynecol Scand 93(8):794–801. https://doi.org/10.1111/aogs.12429
Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B (1996) Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85(7):843–848. https://doi.org/10.1111/j.1651-2227.1996.tb14164.x
Butte NF, King JC (2005) Energy requirements during pregnancy and lactation. Public Health Nutr 8(7A):1010–1027
Mathiesen ER (2016) Pregnancy outcomes in women with diabetes-lessons learned from clinical research: the 2015 Norbert Freinkel Award Lecture. Diabetes Care 39(12):2111–2117. https://doi.org/10.2337/dc16-1647
von Geijer L, Ekelund M (2015) Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep 9(1):224. https://doi.org/10.1186/s13256-015-0709-2
Reader D, Franz MJ (2004) Lactation, diabetes, and nutrition recommendations. Curr Diab Rep 4(5):370–376. https://doi.org/10.1007/s11892-004-0040-6
Roskjaer AB, Andersen JR, Ronneby H, Damm P, Mathiesen ER (2015) Dietary advices on carbohydrate intake for pregnant women with type 1 diabetes. J Matern Fetal Neonatal Med 28(2):229–233. https://doi.org/10.3109/14767058.2014.906577
Greaney DJ, Benson P (2016) Life-threatening lactation or ‘bovineʼ ketoacidosis: a case report. A A Case Rep 7(4):81–84. https://doi.org/10.1213/XAA.0000000000000350
Hudak SK, Overkamp D, Wagner R, Haring HU, Heni M (2015) Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J 14(1):117. https://doi.org/10.1186/s12937-015-0076-2
Feig DS, Donovan LE, Corcoy R et al (2017) Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 390(10110):2347–2359. https://doi.org/10.1016/S0140-6736(17)32400-5
Inkster B, Elder J, Alexander C, Osborne L, Zammitt NN, Frier BM (2015) Post-partum hypogycaemia in lactating women with type 1 diabetes: a pilot study using continuous glucose monitoring. Br J Diab 15:115–122
Acknowledgements
We are grateful to the women who participated in this study. We thank registered nurse M. Glindorf and patient coordinator L. Løndam at the Steno Diabetes Center Copenhagen, Denmark, for practical help during the conduction of this study. Some of the data were presented as an abstract at the 78th ADA Annual Meeting in 2018 and at the 54th EASD Annual Meeting in 2018.
Data availability
Data can be obtained by contacting the corresponding author.
Funding
This research received a grant from the Beckett Foundation. There was no public or commercial funding. Ascensia Diabetes Care Denmark Aps provided the glucometers ContourNext free of charge. The iPro2 Professional CGM devices were purchased at the regular cost negotiated with the Capital Region of Copenhagen.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study, acquired the data and analysed and interpreted data. LR drafted the article. ABR, SE, ALS, HUA, PD and ERM revised drafts critically for important intellectual content. All authors gave final approval of the version to be published. LR is responsible for the integrity of the work as a whole.
Corresponding author
Ethics declarations
HUA owns stocks in Novo Nordisk A/S and participates in advisory boards for AstraZeneca and Novo Nordisk A/S. PD and ERM are participating in multicentre and multinational clinical studies on the use of insulin in pregnant women with pre-existing diabetes in collaboration with Novo Nordisk; no personal honorarium is involved. PD and ERM are in the speaker’s bureau of Novo Nordisk. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ringholm, L., Roskjær, A.B., Engberg, S. et al. Breastfeeding at night is rarely followed by hypoglycaemia in women with type 1 diabetes using carbohydrate counting and flexible insulin therapy. Diabetologia 62, 387–398 (2019). https://doi.org/10.1007/s00125-018-4794-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4794-9